
The Swedish chemist Anders Ekeberg first extracted element 73 - tantalum - from a mineral in 1802, and tantalum was named after its excellent corrosion resistance in acids [1]. Tantalum has superior corrosion resistance to titanium [2-4], is only affected by strong acidic and alkaline environments, and remains relatively inert in the human body [5], thus avoiding interference with biological tissues [1]. By forming bone-like apatite layers in simulated body fluids, tantalum has been shown to be biologically active and to biobind to bone [6]. In addition, pure tantalum and its major oxides, both of which have low solubility and toxicity [7], have been used clinically since 1940 and are widely used in diagnostic and implant applications with significant overall results [8]. Excellent biocompatibility and safety have been shown in orthopaedic, craniofacial and dental related studies [9], and the use of dense tantalum metal implants for osseointegration has been demonstrated to be used for up to 8-12 years [10].
The trend in orthopaedic implants has gradually shifted from fixation to bone by means of cement, mechanical fixation devices and surface attachment to biological fixation by means of osseointegration or bone ingrowth [11-12]. Implantation of porous tantalum into the body provides immediate weight bearing and supports bone deep into the internal structures for good long term fixation [13] and a long term postoperative follow-up study demonstrated a 96% survival rate of porous tantalum acetabular prostheses at year 11 [14].BALLA et al [6] found that porous tantalum showed excellent cell adhesion in MTT analysis and immunochemical studies when compared to porous titanium controls, growth and differentiation, the formation of a rich extracellular matrix on porous tantalum, and a six times higher density of viable cells on the surface compared to the titanium surface.WAUTHLE et al [15] found that porous tantalum has excellent osteoconductive properties, higher normalised fatigue strength and allows more plastic deformation due to its high ductility compared to porous titanium alloys of the same process and structure. Clinical studies have shown that utilising the inward bone growth properties of porous tantalum with suitable structures to achieve stable support and bonding capabilities is an effective way of reconstructing bone defects as the severity and duration of the defect increases [16]. The advantages of porous tantalum implants can be summarised as follows:1) The material has excellent corrosion resistance and biocompatibility.2) Body-matched modulus to avoid stress shielding.3) High surface friction characteristics for improved initial stability.4) Adequate mechanical support and long fatigue life.5) Mimics the mass transport properties of bone to support osteoblast attachment, proliferation, differentiation and mineralisation, possessing excellent osteointegration properties [6,17-18].
The preparation of tantalum as a porous implant requires a reduction in its elastic modulus from 185 GPa to around 3 GPa by increasing the porosity to achieve similarity to human bone (0.4 GPa in trabecular bone to 17.9 GPa in cortical bone) [19-20]. Tantalum is a refractory metallic material with a melting point as high as 2996 °C, and under high melting point, tantalum has high affinity for oxygen and other interstitial elements, so the preparation of porous tantalum with high porosity has a certain technological threshold, and chemical vapour deposition (CVD), powder metallurgy (PM), and additive manufacturing (AM) are the main preparation techniques at present (see Fig. 1 [21-23]).CVD is a complicated and high-cost traditional manufacturing process, porous tantalum implants with patient-specific anatomical features cannot be easily customised, and there are some limitations in the design of the porous structure.PM, although less costly, prepares porous tantalum with lower porosity, uneven pore sizes and high closed porosity, and prominent problems such as internal connectivity, which affects the osteogenic performance of porous tantalum implants. However, a stable and controllable porous structure with high connectivity is an important factor for cell migration, adhesion and proliferation. Therefore, porous tantalum implants prepared using additive manufacturing technology have a controllable and designable pore structure, which is suitable for personalised precision medicine, especially in complex bone defects caused by trauma or tumour resection. Scholars have long believed that the application of tantalum is limited by processing technology rather than biological properties [6,8,24], and relatively high manufacturing costs and quality control difficulties have limited the widespread acceptance of porous tantalum, but emerging metal additive manufacturing is gradually lifting this limitation, and allows for a more flexible and free design of porous tantalum implants in terms of form factor, internal porous structure, and properties, which has greatly facilitated the application of tantalum metal as a biomedical material. medical materials.
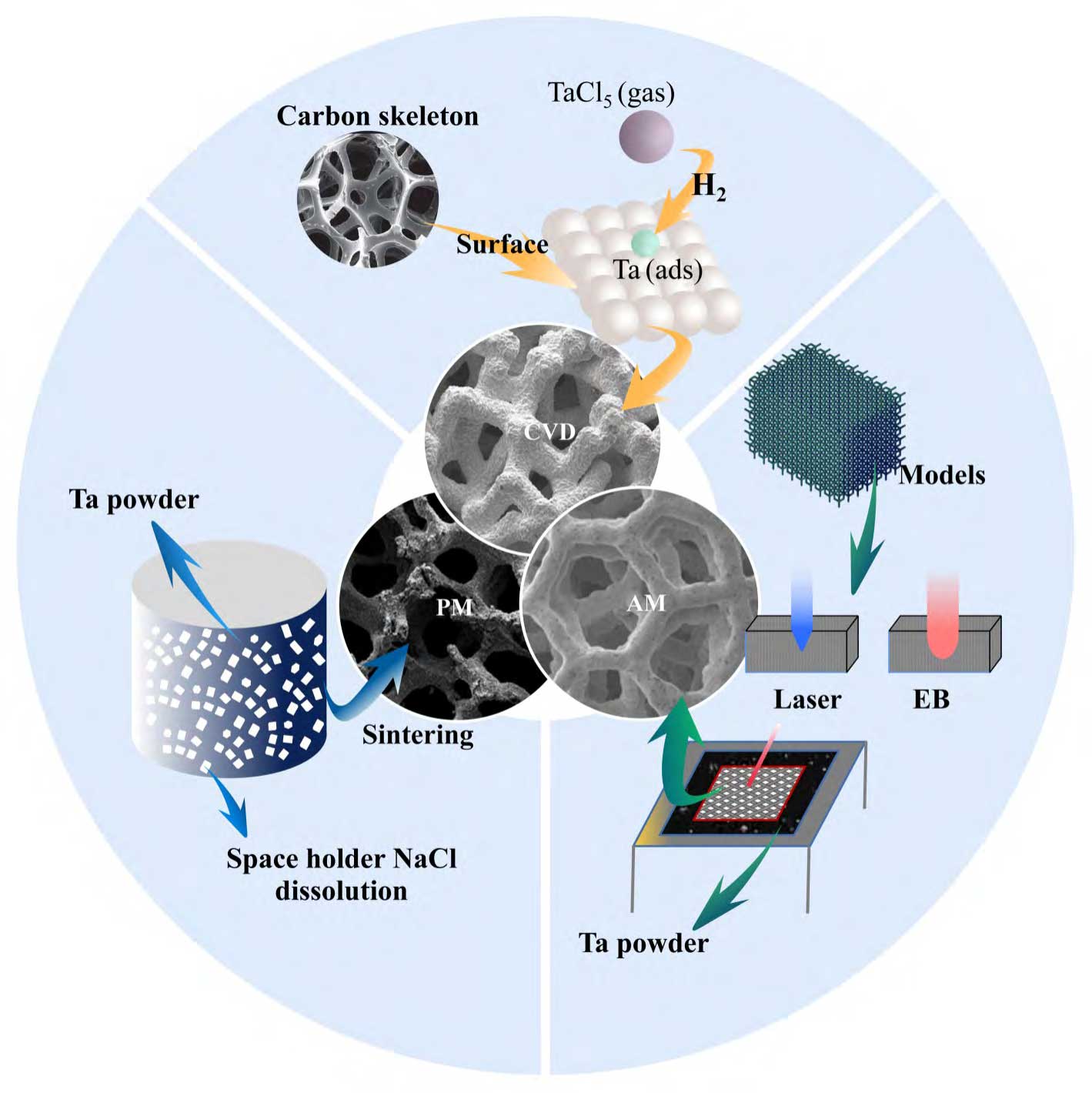
Fig. 1 Main preparation techniques of porous tantalum implants[21-23]
In the additive manufacturing process of porous tantalum implants, from model design, raw materials, equipment to forming process and post-processing will affect the quality of porous tantalum, and the related regulatory science and common technology research work urgently needs to be promoted in a concerted manner. Combined with the actual production, the authors' team summarises the current bottlenecks and difficulties in the preparation of additive manufacturing porous tantalum as follows: 1) In terms of raw materials, the high cost of medical spherical tantalum powder, the low number of times it can be recycled, the lack of research on the key properties of tantalum powder directly affecting the quality of additive manufacturing porous tantalum and the lack of research on the intermediate properties (e.g., spreading properties, humidity), etc., the difficulty and the cost of systematically verifying and controlling the effect of raw material spherical tantalum powder on the physicochemical properties of additive manufacturing porous tantalum. 2) In terms of defect formation, the extremely high cooling rate and non-equilibrium solidification during the additive manufacturing process lead to a variety of uncontrollable defects in porous tantalum, and the defects induced by different process conditions and different levels of interstitial impurity elements differ in shape, size and distribution [25-28]. aliyu et al. [26] studied the formation mechanism of the defects in additively manufactured porous tantalum, including the formation mechanism of the defects in porous tantalum, and the formation of the defects in porous tantalum. defect formation mechanisms, including solidification-induced microporosity, pore-induced microcracking and solidification-induced microcracking, as well as the dislodging of incompletely melted tantalum powder particles from porous tantalum surfaces during post-processing led to the generation of micro-pit and micro-groove defects. Distinguished from other technologies, micro-pit and micro-groove defects have a detrimental effect on the mechanical and biological properties of porous tantalum implants that are currently directly applied to the human body, which is reflected in the absence of local edge positions of porous tantalum wire rods, which are more prone to failure under load.3) In terms of porous tantalum implant particles residue, due to incomplete tantalum powder melting, tantalum powder powder adhesion exists on the surface of porous tantalum wire rods, which is currently lacking in the additive manufacturing porous tantalum cleaning process research, if the removal of tantalum powder particles is not complete, porous tantalum products after implantation in the human body, there is the possibility of particle dislodgement, which will lead to a significant increase in the risk of complications, and also the same impact on the safety and effectiveness of the product [29].
In this paper, the authors reviewed the progress related to the raw materials, preparation technology, mechanical study, ex vivo evaluation and clinical application of additively manufactured porous tantalum, and put forward the future outlook of additively manufactured porous tantalum in combination with the team's many years of research work in customised additively manufactured tantalum metal implants.
1 Raw materials for additively manufactured porous tantalum
Currently, the mainstream porous tantalum additive manufacturing process is powder bed melting, and its raw material is medical spherical tantalum powder in the range of 15~150μm particle size. With the maturity of additive manufacturing equipment and the increased demand for personalized porous tantalum implants, from 2015, research institutions and powder manufacturing enterprises in various countries have carried out the development of the spherical tantalum powder process [30], which has been well controlled in terms of the sphericity of the tantalum powder, the fluidity, the bulk density, the high vibration density, and the rate of hollow powder, etc., and at present, its main preparation technologies are plasma spheronisation (PS) and plasma rotating electrode atomisation (PREP) (see Figure 2 [30-31]). Changes in the initial state of tantalum powder and in the properties of the powder during recycling have a direct impact on the quality of porous tantalum. The quality control measures of tantalum powder and its recycling are one of the technical elements to be considered in the medical device certification process [32]. Currently, YY/T1851-2022 and GB/T38975-2020 are commonly used as acceptable standards for evaluating tantalum powder for powder bed fusion process [33-34].
The principle of PS technology is that high-temperature plasma melts the surface/whole of irregular tantalum powder transported by the carrier gas, and then it condenses into spherical droplets under the action of surface tension, and solidifies into powder in a cooling chamber [31].The raw material used in the PS technology is tantalum powder, and the investment in equipment is large, and the operating costs are high, so the overall cost of preparing spherical tantalum powder is high. Although PS technology spheroidisation is effective, the spheroidisation efficiency is low as well as oxygen contamination is prone to occur during the spheroidisation process.QIN et al [35] reported the existence of a small number of irregular and rough surface particles of spherical tantalum powder prepared by PS technology, and argon was used to protect them during the preparation process, but the oxygen contamination still occurred, and the energy spectroscopy analysis showed that there was an oxide film on the surface of the tantalum powder particles, and the internal oxygen content of the particles was low. Further, HAO et al [36] investigated the effect of raw materials for different processes and process parameters such as carrier gas flow rate, powder feeding rate and secondary spheroidisation on spheroidal tantalum powder, and the results showed that hydrodehydrogenated tantalum powder is more suitable for PS technology than sodium-reduced tantalum powder. The quality of spherical tantalum powder is ultimately reflected in the quality of additive manufacturing tantalum formers, the oxygen content of tantalum powder is an important factor affecting the microstructure and mechanical properties of additive manufacturing tantalum formers.SUNGAIL et al [37] prepared high and low oxygen content tantalum powder formers and carried out microstructural observation and mechanical property tests, the results show that the oxygen content of tantalum powder with low oxygen content (87 × 10-6 ) formers with low oxygen content (107 × 10-6 ) and low oxygen content (107 × 10-6 ) are more suitable for PS technology. low oxygen content (107 × 10-6 ), with high elongation and tensile strength (35%, 634 MPa), and the tensile fracture mode was ductile fracture.SHI et al [38] used 481 × 10-6 tantalum powder to prepare the formed parts with a maximum tensile strength of 697 MPa, and the elongation rate was 28.5%. Tantalum powders prepared by fluidisation, air flow milling (JM) and PS techniques, and found that due to the low oxygen content of tantalum powders prepared by JM and PS techniques, the formed parts showed good precision and low hardness.TAN et al [40] investigated the effect of oxygen content of tantalum powders on the additive manufacturing process, microstructure, and mechanical properties, and the results showed that the process window of tantalum powders with low oxygen content was larger, and with the increase of the oxygen content (150×10-6→470×10-6), the formed parts were more precise than the formed parts, with the increase of the oxygen content (150×10-6→470×10-6). 10-6), the grain structure of the formed parts changed from {100}||BD to {111}||BD and the ultimate tensile strength increased (391 MP (42% → 29%). The difference between the findings of SUNGAIL and TAN on the elongation of the formed parts may be influenced by the study interval of the oxygen content of tantalum powder (SUNGAIL: 87 × 10-6~829 × 10-6, TAN: 150 × 10-6~470 × 10-6), the forming equipment and the process.
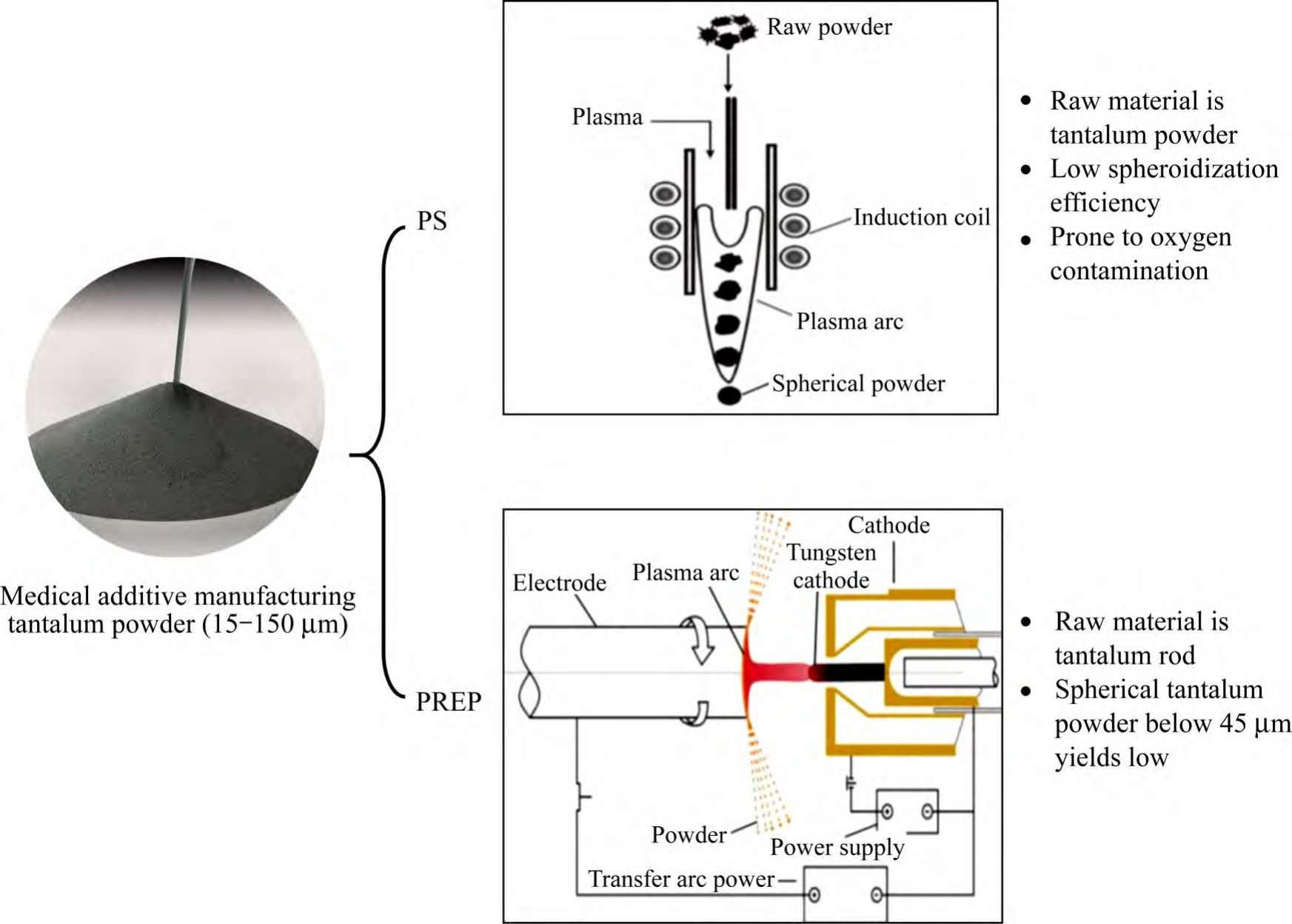
Fig. 2 The main preparation technologies and difficulties of spherical tantalum powder for medical additive manufacturing
The principle of PREP technology is that high-temperature plasma melts the end face of the tantalum rods of the self-consuming electrodes rotating at high speed, and the molten liquid film is broken into micro-droplets under the effect of centrifugal force and solidified into powder [31], compared with PS technology, PREP technology is based on high-purity tantalum rods as raw materials, which has obvious advantages in the cost of production, the control of powder morphology and the content of impurities [30]. However, the yield of spherical tantalum powder below 45 μm prepared by PREP technology is low, generally less than 10%. Therefore, improving the fine powder yield of PREP tantalum powder is the main challenge for this technology in the field of porous tantalum additive manufacturing.
In addition to the previous focus on additive manufacturing process parameters, recent studies have shown that factors such as the content of interstitial impurity elements in tantalum powder have a significant impact on the biological properties of porous tantalum, the stability of the additive manufacturing process and the quality of the subsequent implant. On the one hand, interstitial impurity elements such as oxygen have a strong solid solution strengthening effect on BCC-structured tantalum, which has a significant effect on the toughness of tantalum [41], and a small amount of interstitial oxygen atoms is beneficial to the improvement of tantalum toughness [42]. On the other hand, the increase in oxygen content reduces the wetting and diffusion ability of the melt and hinders the connection of the melt pool, leading to partial melt pool instability [40]. The densities of low-oxygen tantalum powder (150 × 10-6 ) formed parts were insensitive to the laser energy density, whereas the high-oxygen tantalum powder (470 × 10-6 ) formed parts still showed an increase in the number of pore defects and larger sizes under the optimised process parameter conditions [40], which suggests that the increase in the oxygen content resulted in a narrower process window. As the initial oxygen content of tantalum powder increased, along-crystal fracture was observed in the samples with an oxygen content of about 510 × 10-6 at the fracture location, and due to the enrichment of oxygen elements at the grain boundaries [41], the main fracture mode was changed from the typical ductile fracture to a combined fracture mode of brittle and ductile fracture [37]. Tantalum powder impurity elemental oxygen mainly originates from gases encapsulated in the powder [41]. According to the above domestic and international studies [31, 37-38], with the increase of oxygen content in the raw tantalum powder, the oxygen increment of tantalum for additive manufacturing shows an overall increasing trend, which is mainly due to the following two reasons: 1) The increase of oxygen content (oxides) in the raw tantalum powder leads to a higher melting energy required, and the long residence time of the melt pool under the action of the high energy density results in a slower solidification speed, and the diffusion of the surface oxygen to the inside of tantalum formed parts takes a time becomes longer.2) Marangoni convection is more pronounced, leading to an increase in the contact area of the sputtered tantalum powder particles with the oxygen impurities in the forming chamber atmosphere.LEI et al [43] showed that ex vivo and in vivo tests showed better bone formation due to the low contact angle of low-oxygen tantalum water. Therefore, tantalum powder with too high oxygen content is not favourable for the preparation process and clinical application of additively manufactured porous tantalum (see Figure 3 [35,40,42]).
Tantalum powder is relatively expensive and recycling it in the additive manufacturing process can reduce the cost. Metal powder recycling is a process state unique to additive manufacturing technologies that use metal powder as a raw material, and recycling can greatly improve powder utilisation. Additive manufacturing forming environment, powder recycling, sieving, drying, mixing, storage and other aspects of the process of multiple use may introduce impurity elements, which will have a certain impact on the quality of the powder. The Guiding Principles for Research on the Homogeneity of Physicochemical Properties of Additively Manufactured Metal Implants (No. 4, 2022) mention the need to validate the impact of recycled powder on the homogeneity of the printing process and product quality. In order to validate the number of times tantalum powder can be recycled, the authors' team systematically investigated the change of powder properties during the recycling process of additively manufactured tantalum powder and its impact on the quality of tantalum formed parts [44]. In terms of powder properties, as the number of tantalum powder recycling times increased, the oxygen content of tantalum powder gradually increased from 46 × 10-6 initially to 180 × 10-6 in 30 furnaces of recycling. due to the forming in a vacuum environment, the oxygen increment of tantalum powder in a single furnace was only 4.7 × 10-6 on average, and the particle size of tantalum powder slightly increased due to spattering, adherence, and remelting in the process of forming and due to the sintering connection, crushing recovery of the powder particles during the recycling process. Due to the sintering connection and crushing and recycling of the powder particles in the recycling process, the surface morphology of the powder particles became incomplete, so the fluidity decreased slightly, but the bulk density and vibration density did not change significantly. In terms of the properties of the formed parts, the following conclusions were made under the same process parameters: 1) The study of solid tantalum showed that there was no introduction of impurities during the reuse of tantalum powder, with a decreasing trend in the densification, and no significant change in the tensile strength, whereas the elongation decreased with the increase in the number of times of reuse, with a decrease in elongation of 34.7 per cent after 30 times of reuse. 2) The study of porous tantalum showed that, after 30 times of reuse of the tantalum powder, the porous tantalum porosity remained stable, but the surface spheroidisation increased (see Fig. 4 [44]), and the compressive yield strength and plateau stress both decreased by about 20%. Taken together, it is particularly critical to pay attention to the oxygen content of the raw material tantalum powder in the additive manufacturing process of porous tantalum.
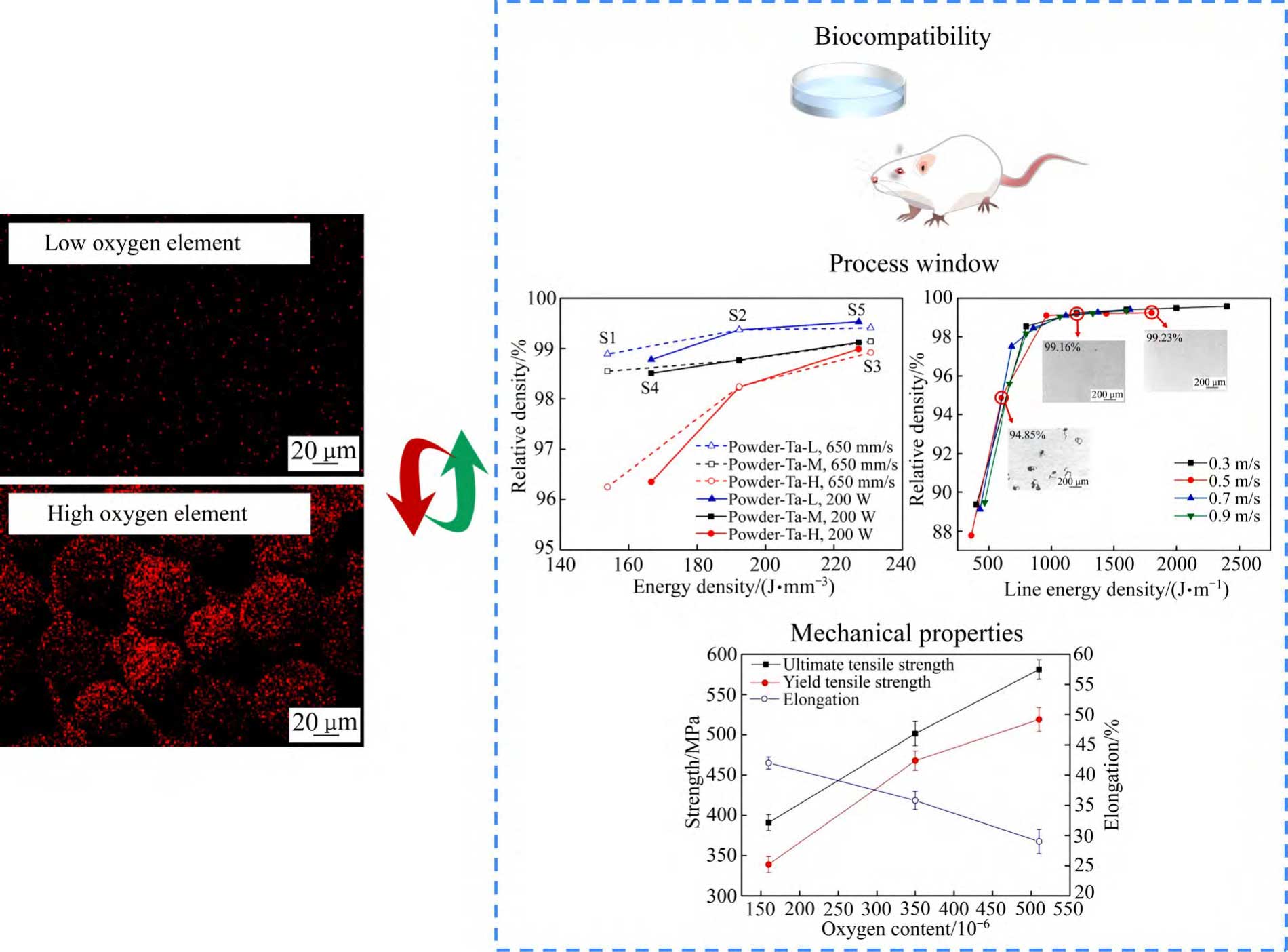
Fig. 3 Oxygen content of tantalum powder affects biocompatibility, process window and mechanical properties of additive manufacturing porous tantalum
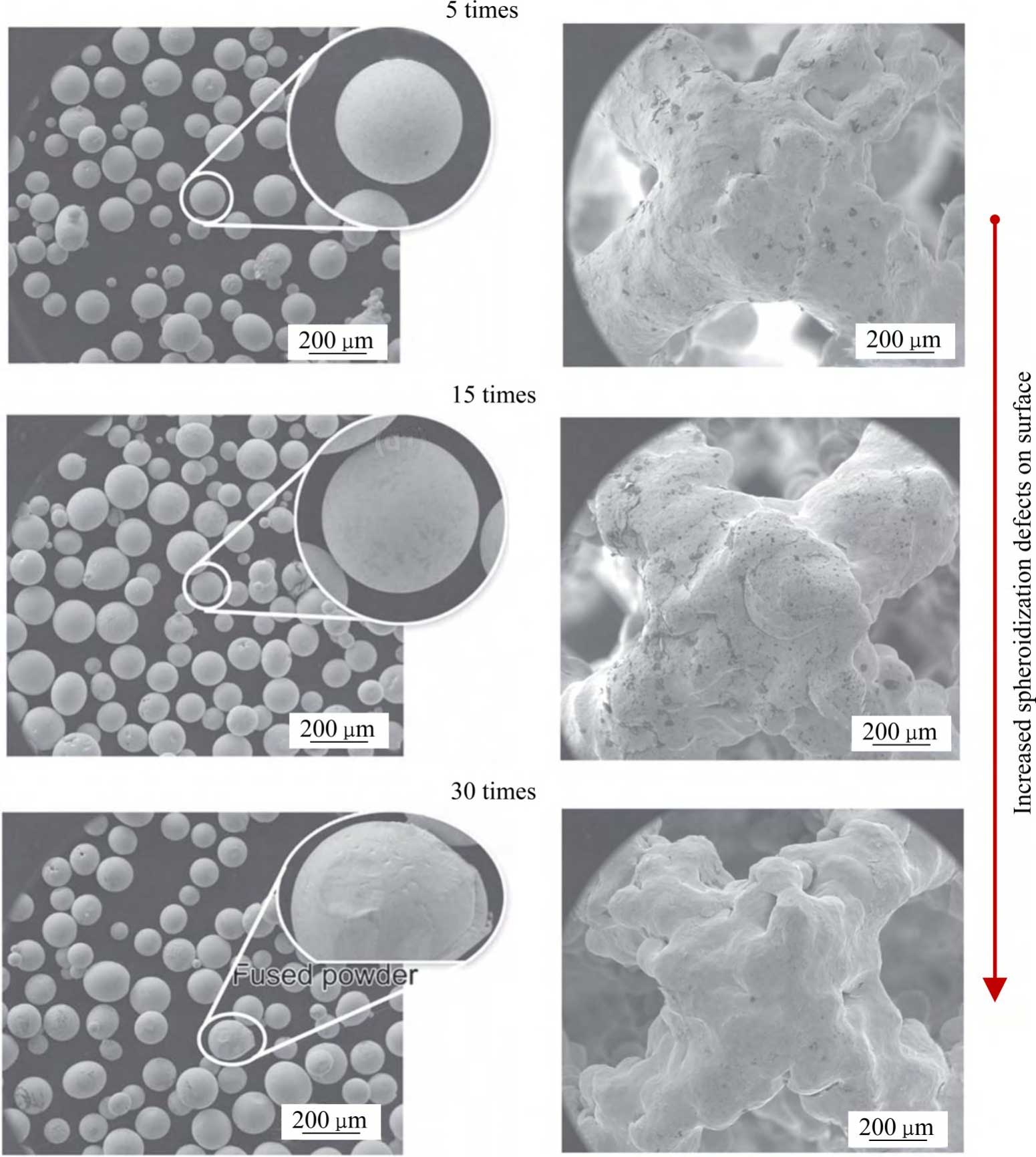
Fig. 4 Effects of reuse 30 times on surfaces of tantalum powders and additively manufactured porous tantalum[44]
2 Additive Manufacturing of Porous Tantalum
In clinical treatment, through medical-industrial interaction, additive manufacturing technology can be used to precisely customise orthopaedic implants with patient-specific anatomical features, and at the same time, the pore structure parameters of different regions of the implant can be customised to meet the elasticity modulus requirements of human bone tissues and avoid the phenomenon of stress shielding. Additive manufacturing is a process of manufacturing parts or physical objects by stacking materials based on 3D model data. According to the technical principles, GB/T35021-2018 and ISO/ASTM52900:2023 classify the current additive manufacturing technologies into seven categories [45-46]. Among them, the selective laser/electron beam melting technology (SLM/EBM) is the most widely used technology in the additive manufacturing of metallic orthopaedic implants due to its advantages of high precision, high efficiency, and good stability, and it belongs to the powder bed fusion process (PBF) in the classification.
2.1 SLM technology
The process flow and technical principle of preparing porous tantalum implants using SLM technology is shown in Fig. 5 [13,44,47], where a high-energy laser beam with suitable parameters in the additive device selectively melts the tantalum powder laid on the substrate, and porous tantalum shaped parts with controllable apertures are fabricated layer by layer according to the data of the porous structure model, and the whole shaping process is carried out in a protective atmosphere to avoid oxidisation, and the shaped parts are used to be The porous tantalum molded parts are cut from the substrate using a cutting machine, and the unmelted powder inside the porous tantalum molded parts is removed by sand blasting and other processes, and then the molded parts are annealed to eliminate the residual stresses generated during processing, and the final porous tantalum implant is obtained by cleaning and sterilisation and packaging. During the preparation process, the porous structure model data is obtained by engineers who design the implant and process the model prior to printing.
The particle size range of the raw material tantalum powder suitable for SLM technology is typically 15-53 μm.
During SLM forming of tantalum metal, complex thermal processes lead to differences in the microstructure of tantalum formed parts with different morphologies [22,48-53]: 1) Directional melting and rapid solidification due to thermal inputs at different process parameters. 2) Scanning strategy. 3) Partial remelting of the previous deposited layer. 4) Differences in the thermal conductivity of the substrate material, as well as substrate-induced specific directions of the heat flow, etc. SLM Forming The microstructure of bulk dense tantalum usually has strongly columnar grains along the forming direction, with an average grain size of about 54.7 μm, which is larger than the average grain size of conventionally forged tantalum (about 18.9 μm) (see Fig. 6(a) and (c) [54]).The grain shape of SLM porous tantalum is close to that of SLM bulk dense tantalum, and since the filament diameter of porous tantalum is usually in the range of several hundreds of micrometres, the heat diffuses into the powder more readily, the The grain size is closer to that of wrought tantalum (see Fig. 6(b) [55]).
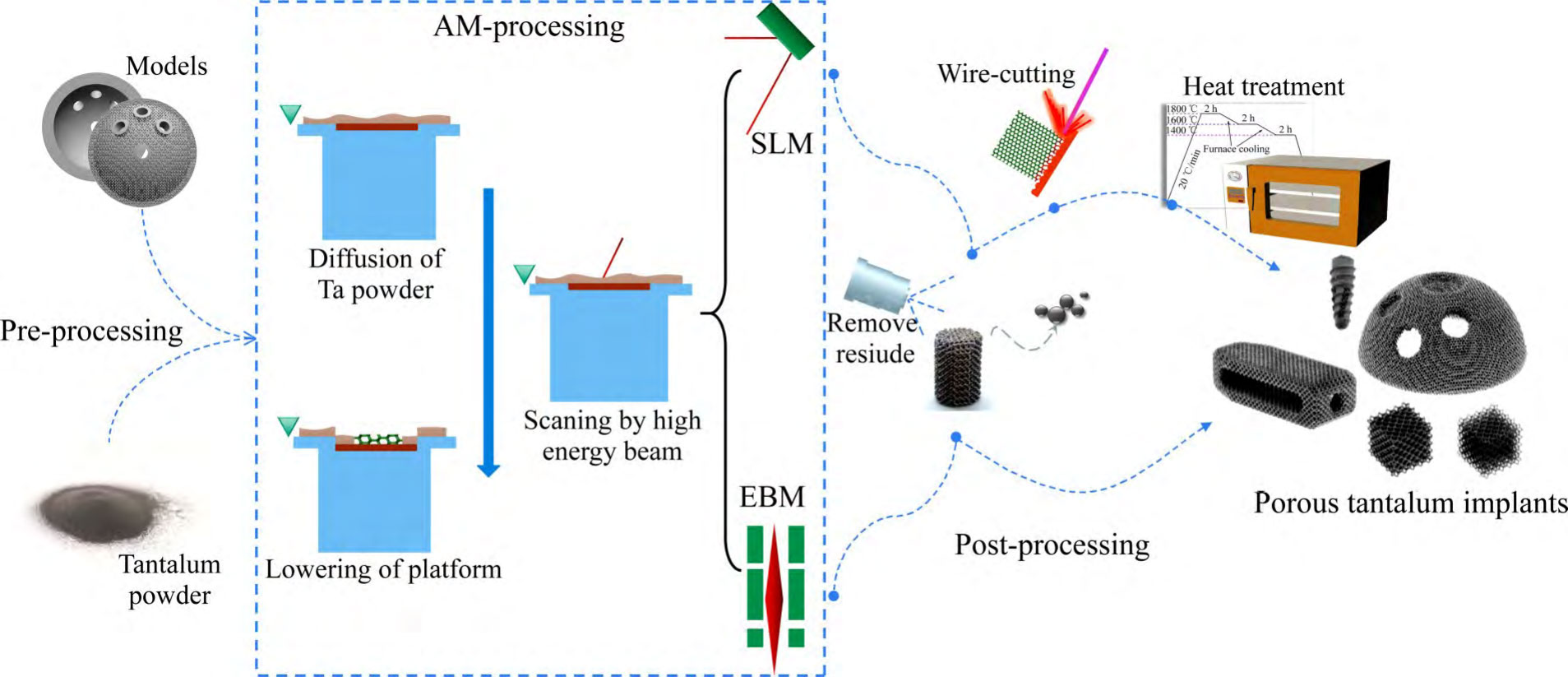
Fig. 5 Process flow and technical principle of SLM and EBM technology to prepare porous tantalum implants
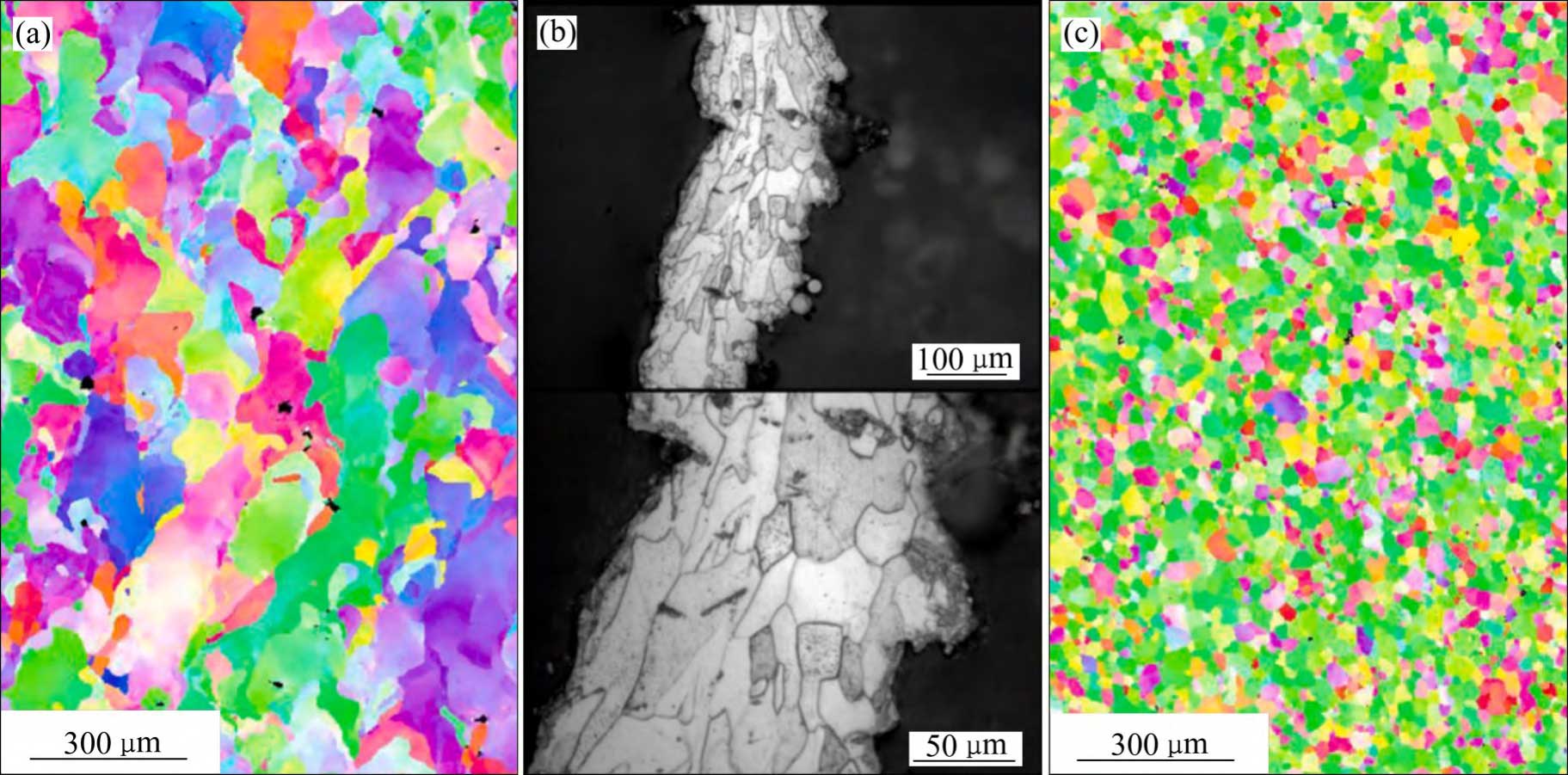
Fig. 6 Microstructures of tantalum[54-55] : (a) SLMed massive dense tantalum; (b) SLMed porous tantalum; (c) Forged tantalum
The unique microstructure and surface micro- and nanostructural features of additively manufactured porous tantalum cause changes in wettability, surface energy, and biological properties more favourable for clinical applications.CHEN et al [54] reported that the adhesion and proliferation of rat bone marrow stromal cells were enhanced in SLM tantalum samples as compared with the conventional forged tantalum control group, and the reason for this may be related to the higher surface energy of the SLM tantalum samples (64.42 mN/ m) and lower contact angle (Distilledwater40.03°) of SLM tantalum samples. According to VITOS et al [56], the surface energy of tantalum shows an increasing trend from (110), (100), (211), (310) to (111).In the study of CHEN et al [54], the wrought tantalum is dominated by (110), and the SLM tantalum is distributed in the direction of (110), (211), and (310).YANG et al [57] prepared by the SLM technique the trabecular tantalum scaffolds and compared them with the CVD trabecular tantalum scaffolds from Jetmax in the U.S. The results showed that the porous structure and mechanical properties of the two were similar, so there is a prospect of additively fabricated porous tantalum for bone filling and reconstruction applications, and the SEM observation further showed that both filaments have micro- and nanostructured surfaces, but the filaments and pore sizes of the former are larger, so in the preparation of biomedical fine porous structure implants, additive manufacturing equipment and processes can be further optimised.
2.2 EBM technology
The process and technology principle of EBM technology for preparing porous tantalum implants is similar to that of SLM technology, with the following differences: 1) The heat source used is an electron beam, and the forming process is carried out in a vacuum. 2) The particle size of tantalum powder, which is commonly used as a raw material for EBM, generally ranges from 45~105 μm or 45~150 μm. 3) The forming process includes a preheating and thermal compensation process, and therefore the porous tantalum formed parts are usually The support is simple and can be disassembled directly without the need for subsequent wire cutting, heat treatment and other processes, which is conducive to a rapid response to the demand for personalised implants.
Due to the added substrate preheating, powder pre-sintering and interlayer thermal compensation processes during the forming process, the microstructure of EBM-formed bulk dense tantalum has more intense columnar grains along the forming direction (see Fig. 7(a) [53]), increased average grain size (~89.5 μm) (see Fig. 7(b) [42]), excellent plasticity, and elongation up to 46% ± 12% compared to the SLM technique [52]. There is a lack of research related to the microstructure of EBM-formed porous tantalum.
2.3 Other technologies
Based on the demand for porous tantalum with reduced cost or better biological properties, researchers have attempted to make breakthroughs in other additive manufacturing methods. Directed energy deposition by laser (DED-L) obtains porous tantalum by simultaneous melting and deposition of tantalum powder using a high-energy laser beam.BALLA et al [6] prepared porous tantalum with porosity of 27% and 55% respectively, and compared with porous titanium, which verified that porous tantalum has a better cell adhesion, growth and differentiation.CHEN et al [58-59] prepared porous tantalum samples with porosity ranging from 35.48%~50 of porous tantalum samples, due to factors such as the large spot diameter of the laser beam used in the current process led to the low precision of porous tantalum fabrication, which limited its application in small aperture high porosity porous tantalum. Compared to powder beds, the tantalum powder feedstock for the DED-L technology is transported via carrier gas, so the equipment requires low powder storage capacity and tantalum powder is less likely to accumulate inside the porous interior, which is a great advantage in terms of cost savings and reducing the risk of residual powder.
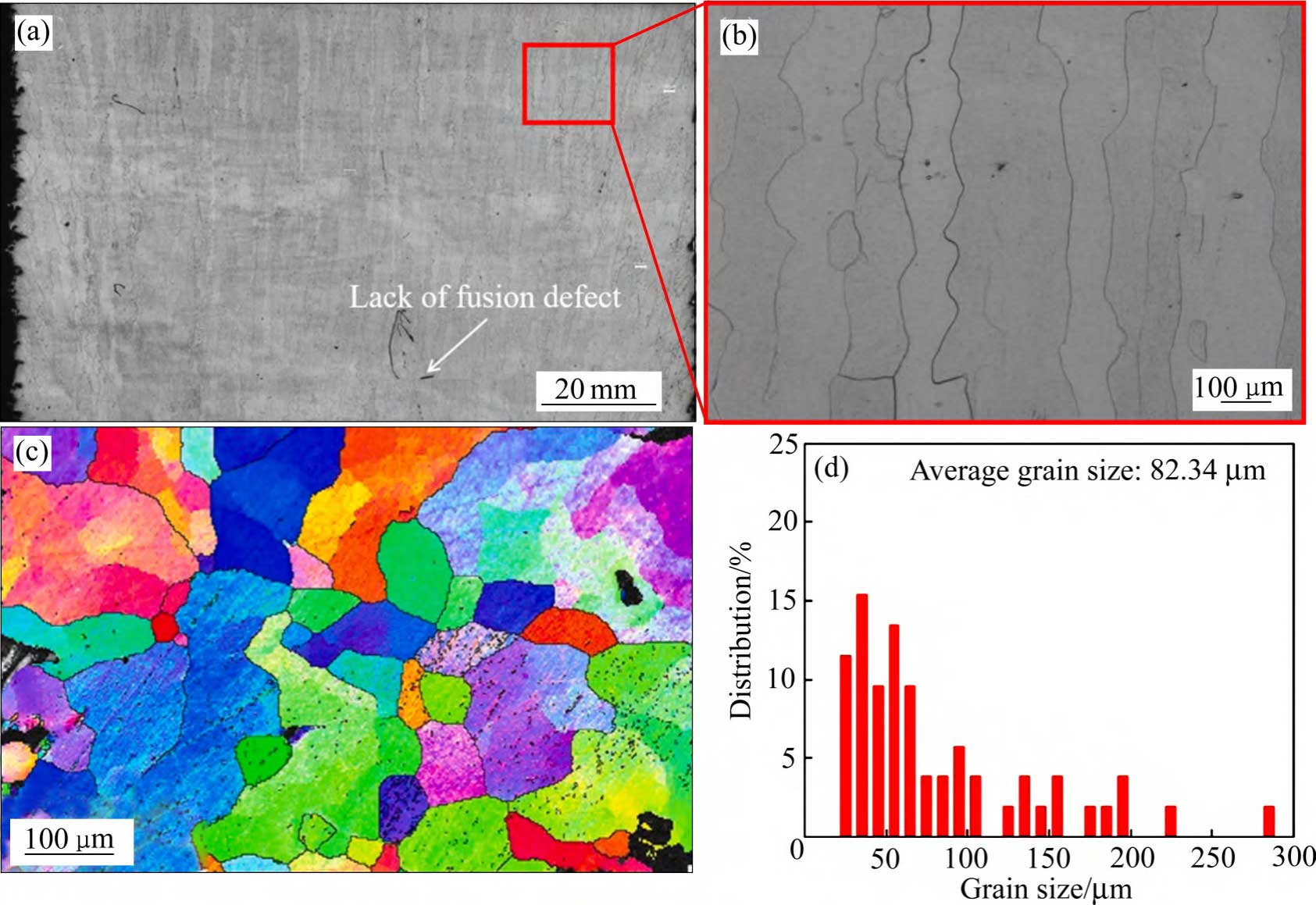
Fig. 7 Characteristics of preparation of dense tantalum samples by EBM technology[42, 53] : (a), (b) OM image; (c) EBSD inverse pole figures; (d) Grain size distribution
Hybrid manufacturing methods of additive manufacturing combined with other technologies are favourable for the development of low-cost, high-performance and multifunctional porous tantalum implants.WANG et al [60] deposited tantalum coatings on SLM porous titanium alloy scaffolds using a CVD process and verified that tantalum coatings could improve the biological properties of additively manufactured porous titanium alloys through in vitro and in vivo studies.FOX et al [61] used SLM technology to form porous tantalum coatings on cobalt-chromium alloy surface with a porous tantalum coating, and the interface between the two metallic materials was characterised by microstructure without cracks. Implants were prepared by depositing porous tantalum on a base plate of a different metallic material, similar to the current grafted additive manufacturing scheme in form-following cooled moulds, where the base is usually completed by conventional machining, resulting in cost reduction and efficiency.ZHAO et al [62] developed a hybrid method based on the fabrication of additive manufacturing gel-irrigated Ta2O5 precursor, followed by electrolytic reduction for the preparation of tantalum metal stents. The results showed that the prepared tantalum scaffolds were non-toxic and promoted cell adhesion and proliferation in vitro. Primary macropores were formed by gel infusion, which provided controlled interconnected pore channels for new bone formation and growth, and then secondary micropores were prepared by electrolytic reduction method with the aim of promoting cell adhesion, proliferation and migration, and the XRD results showed that Ta2O5 was reduced to pure tantalum, but the compressive strength was lower than 5 MPa.The method utilises cheap tantalum oxide powder as the initial raw material, which has a relatively low cost, but further optimisation is still required! The mechanical properties of porous tantalum scaffolds can meet the clinical needs.
3 Mechanical and biological research of additive manufacturing porous tantalum
3.1 Mechanical properties
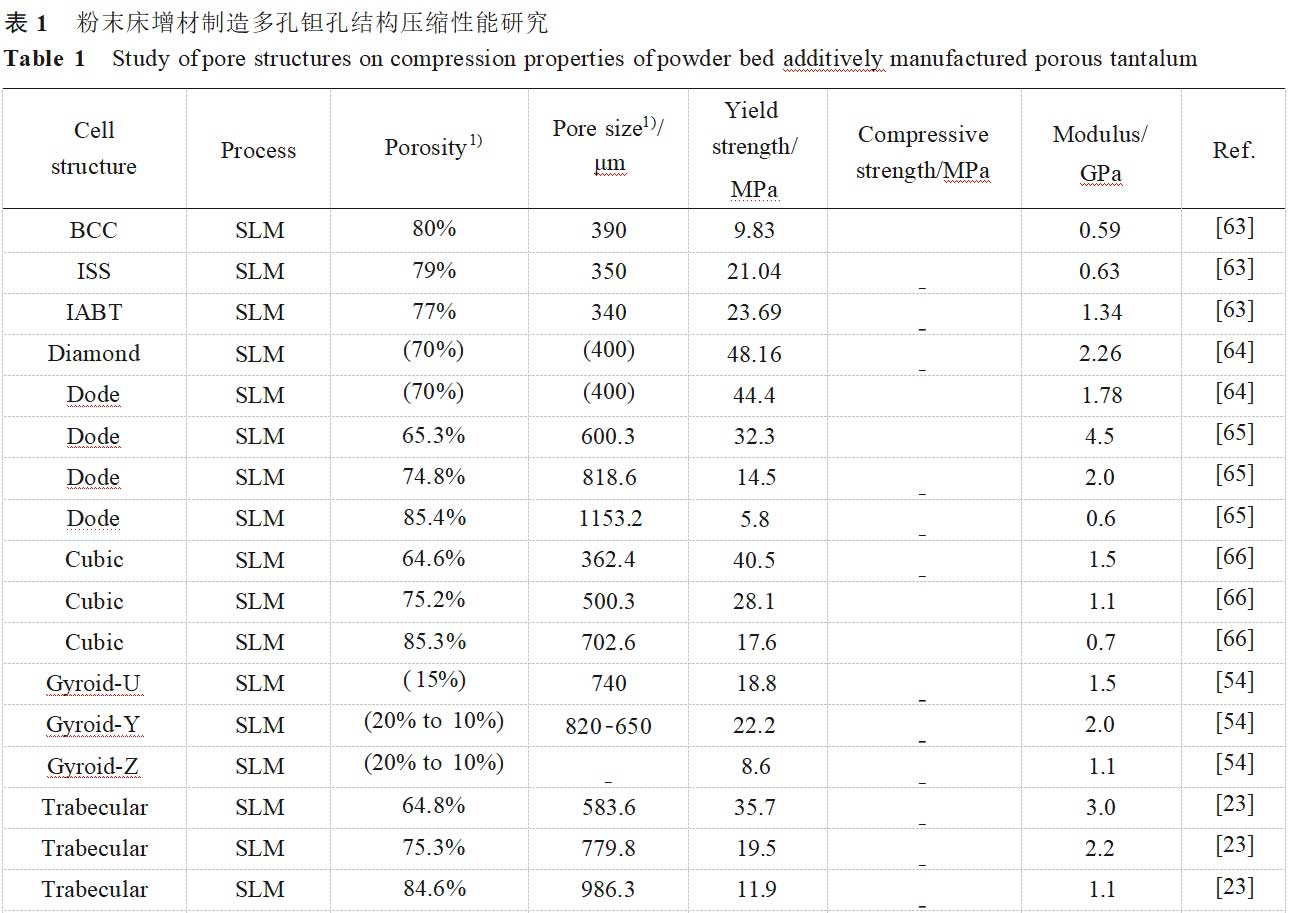
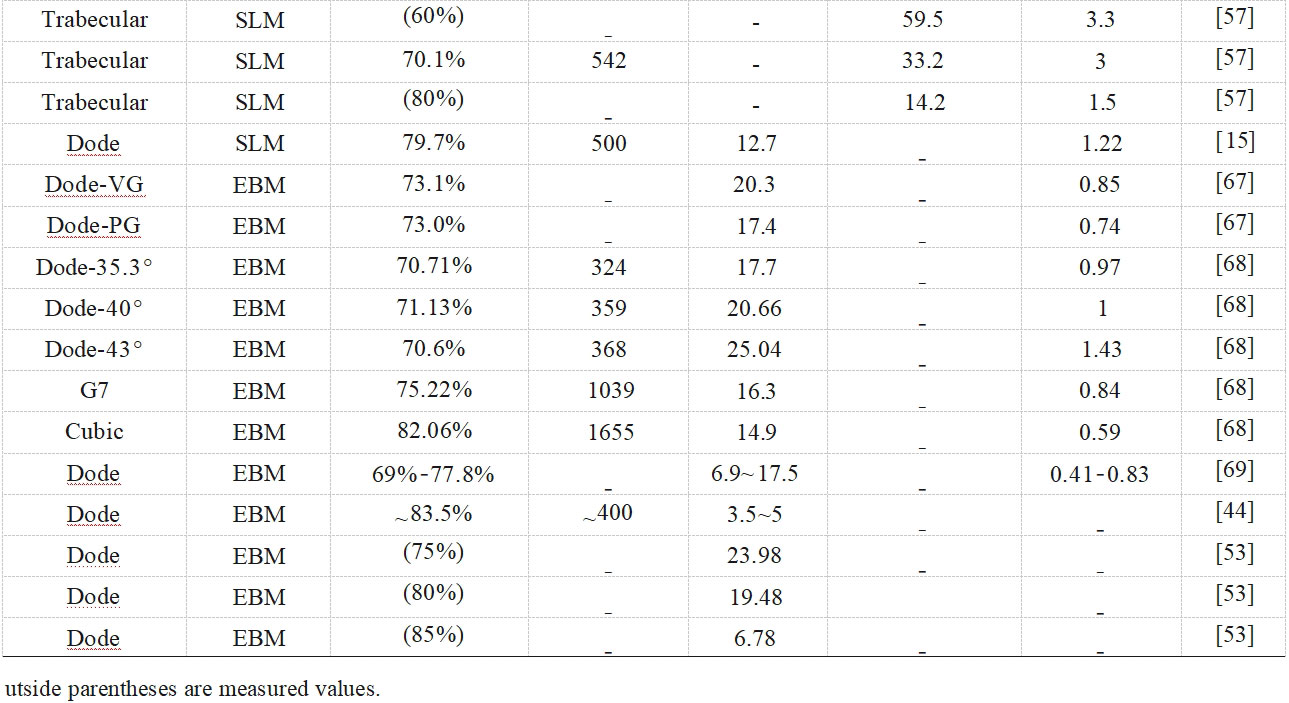
The mechanical properties of biomedical porous materials significantly affect the growth of new bone, osseointegration and physical function replacement of bone defects [57], and porous tantalum should have sufficient yield strength and low modulus of elasticity to adapt to the mechanical response of the bone tissue and reduce stress shielding [63]. In orthopaedic applications, especially in the field of load-bearing bone prostheses, static compression properties are important mechanical properties for evaluating porous materials, and Table 1 [15,23,44,53-54,57,63-69] summarises the compression properties studies of porous tantalum pore structures fabricated by powder bed additive manufacturing. Over the past decades, many models have been developed to relate porous structures and mechanical properties, with factors such as connection type and porosity influencing the applicability of the theoretical models [70].The Gibson-Ashby model is a classical model commonly used for designing and predicting the mechanical properties of porous structures, and the relationship between the modulus of elasticity (E and Es), the strength (σ and σs), and the relative density (ρ/ρs) are related as follows [71]:
E/Es = C1 ( ρ/ρ s )n (1)
σ/σs = C2 ( ρ/ρ s )n′ (2)
Where: the subscript s denotes the fully dense material; C1 and C2 are constants related to the unit-pillar material; n and n' are the exponential factors.
Due to the presence of incompletely fused powder on the surface, surface step effects and internal defects in additively manufactured porous metallic materials, the accuracy of the model in predicting the mechanical properties will be affected to some extent. It has been found that the modulus of elasticity is more affected by the pore structure fillet than porosity [72], and the actual strength is usually lower than that in the ideal state [69]. Combined with the consideration of raw materials and additive manufacturing processes in the above sections, the mechanical properties of porous tantalum may be affected by the following aspects [73-79]: 1) Mechanical properties of the tantalum itself, including elastic modulus, yield strength and ultimate strength, etc. 2) Design of porous tantalum pore structure, including pore unit morphology, unit orientation, and the combination of unit arrangement, etc. 3) Fabricability, including defects and lattice features fidelity, etc. 4) Pore structure design, including pore unit morphology, unit orientation, and unit combination alignment, etc. 4) Additive manufacturing forming direction, placement direction and preparation furnace/batch etc. 5) Heat treatment, surface treatment etc. Overall, the lower modulus of porous tantalum implies high porosity and suitable filament morphology, and is generally accompanied by reduced strength. For porous tantalum implants, the high porosity is also beneficial for weight reduction and patient comfort due to the high density of tantalum (16.6 g/cm3).
In the mechanical study of SLM porous tantalum, YANG et al [23,47,65-66] investigated the effects of three pore structures, cubic (Cubic), rhombic dodecahedron (Dode), and bionic bone trabecular (Trabecular), and their porosities on the static compression behaviour of porous tantalum (see Fig. 8 [23,65-66]): 1) Compression of porous tantalum with three pore structures strength increases with decreasing porosity.2) The failure mechanism of the three pore-structured porous tantalum materials is plastic deformation and fracture of the filament rod, therefore, the mechanical reliability of the three pore-structured porous tantalums is better than that of porous titanium alloys, with cubic corresponding to the strain at the yield point being the largest and the strongest resistance to deformation, bionic bone trabeculae the second largest, and rhombic dodecahedron the lowest.3) The maximum stress of cubic mainly distributes in the longitudinal filament rod, bionic bone trabeculae and rhombic dodecahedron are mainly distributed at the intersection of the filaments, i.e. the failure position. At the same time, they studied the dynamic compression behaviour of porous tantalum with uniform, y-gradient and z-gradient structures. y-gradient structure showed the best plateau stress and compression modulus among the three different gradient structures due to the largest local volume fraction on the fracture surface, and y-gradient was also superior to the other two structures in the fatigue response at different stresses.
In terms of porous tantalum structural innovation, fine control of the mechanical properties of the porous structure through cell topology is crucial for the design of long-life personalised porous tantalum implants.LI et al [80] prepared two types of porous tantalum scaffolds (BCC and topologically BCC-D) by using SLM technique. Among them, the mechanical properties of BCC-D are anisotropic, while BCC porous tantalum has almost the same mechanical properties in the forming direction and horizontal direction, with elastic modulus and yield strength close to 1.9 GPa and 56 MPa, respectively.As shown in Fig. 9 [63], ZHANG et al. [63] based on the inspiration of the SLM technique and the saddle, innovatively designed and prepared a reduced elastic modulus without loss of strength of ISS structure, and used a combination of experimental and finite element analysis to explore the mechanical properties, deformation mechanism and energy absorption characteristics of porous tantalum with the novel ISS structure, and concluded that the ISS lattice has a promising application in load-bearing orthopaedic implantation.
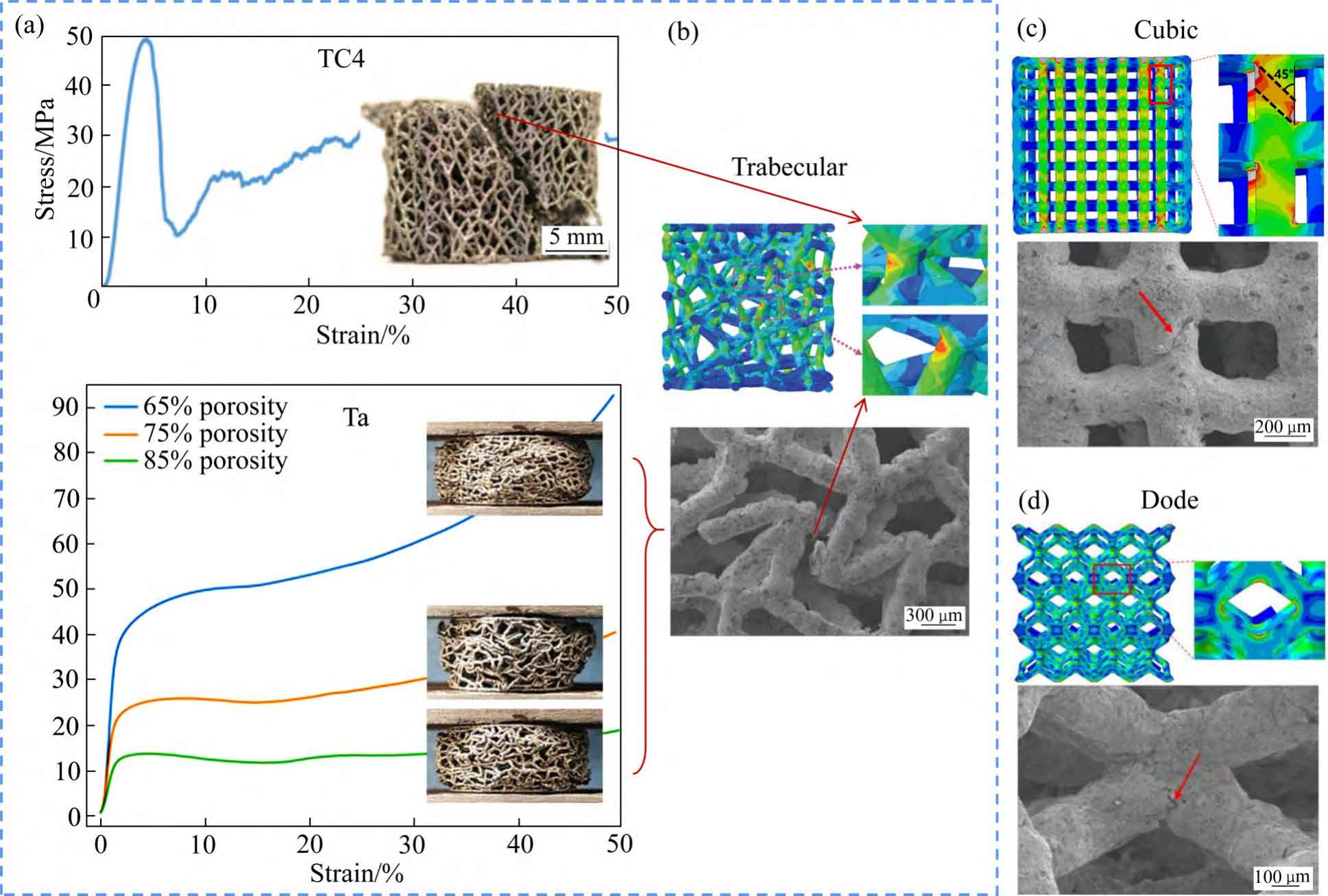
Fig. 8 Comparison of compressive properties of Trabecular structured porous titanium alloy and porous tantalum(a) and failure points of three pore structures of Trabecular(b), Cubic(c) and Dode(d)
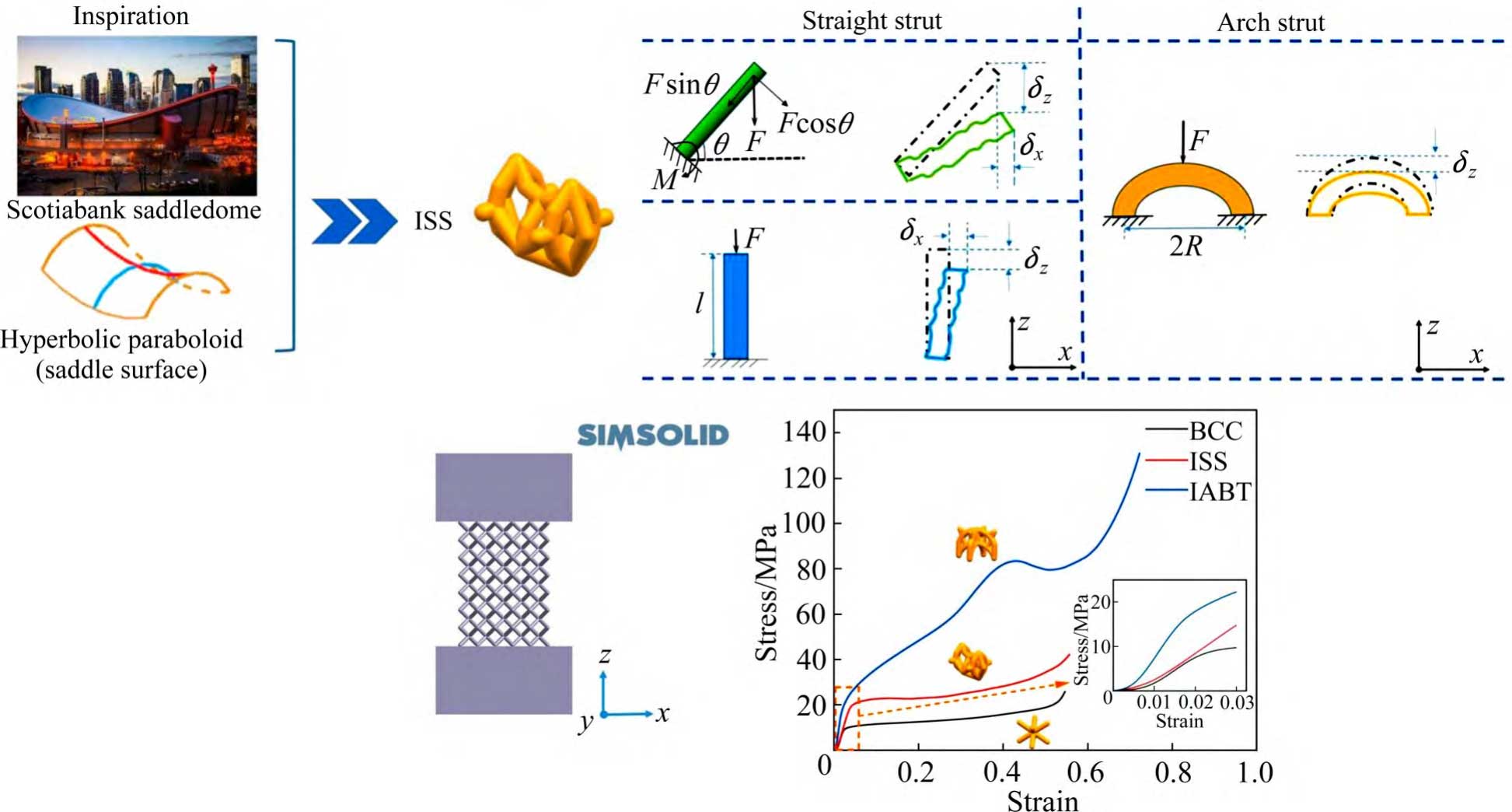
Fig. 9 Schematic diagram of elasticity modulus reduce of ISS structure designed without loss of strength
In the mechanical study of EBM porous tantalum, the authors' team [67-69,81] investigated the effects of various structures and their porosity on dynamic and static compression and static tensile and flexural properties. In compression tests, G7 and cubic porous tantalum were dominated by the flexural deformation of the filament rod, while rhombic dodecahedron had higher compressive fatigue strength and fatigue ratios than rhombic dodecahedron porous tantalum due to the presence of the vertical struts, and the fatigue ratio was the highest for cubic porous tantalum, and when the angle of the rhombic dodecahedron porous tantalum filament rod was increased from 35.3° to 43°, through the increase of the flexural deformation, the rhombic dodecahedral porous tantalum fatigue strength and fatigue ratio increased by 59% and 20%, respectively. In addition, in the tensile and bending tests, the maximum stress of rhombic dodecahedral porous tantalum was uniformly distributed, and the uniform structural deformation delayed the fracture of the filament rods, while G7 and cubic porous tantalums made the filament rods fracture at the early stage of the deformation due to the concentration of the stress on some of the positions. Based on the plotting of compression, tensile and bending property test results of porous tantalum samples with different porosities (see Fig. 10 [69]), the relationship between the mechanical properties of porous tantalum and its relative density is derived as follows:
E = 9.6(ρ/ρ s )2.05 (3)
σ 1 = 546.98(ρ/ρ s )2.9 (4)
σ2 = 613.93(ρ/ρ s )2.39 (5)
σ3 = 3262.11(ρ/ρ s )4.51 (6)
Where: σ1, σ2 and σ3 denote the compressive yield strength, flexural yield strength and tensile yield strength, respectively, which are linearly related to the relative density with the exponential factors n′ of 2.9, 2.39 and 4.51, respectively, and the modulus of elasticity and the relative density with the exponential factor n of 2.05, which is in better agreement with the Gibson-Assby model.
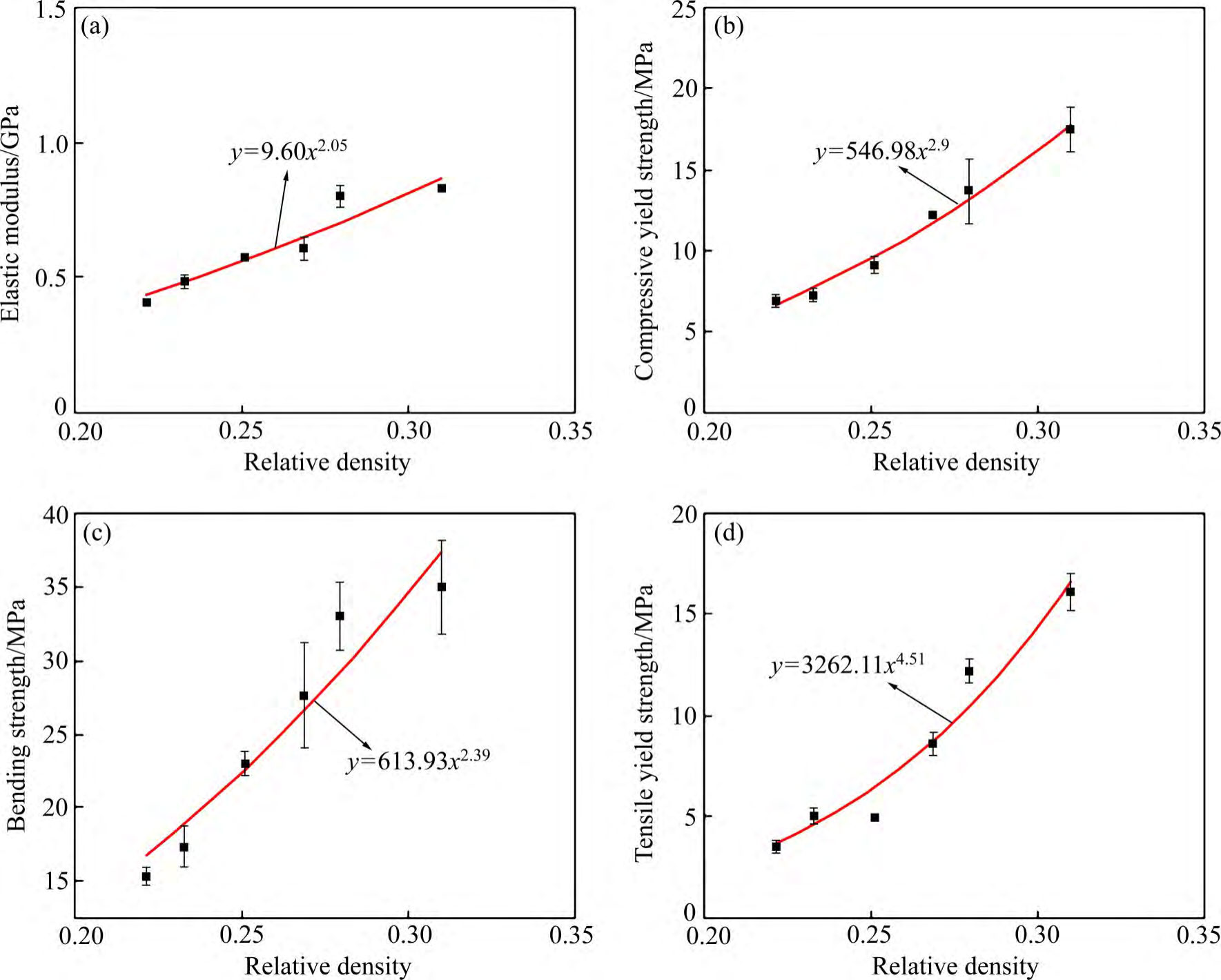
Fig. 10 Relationship between mechanical properties and relative density for porous tantalum scaffolds[69] : (a) Plots of elastic modulus; (b) Compressive yield strength; (c) Bending strength; (d) Tensile yield strength
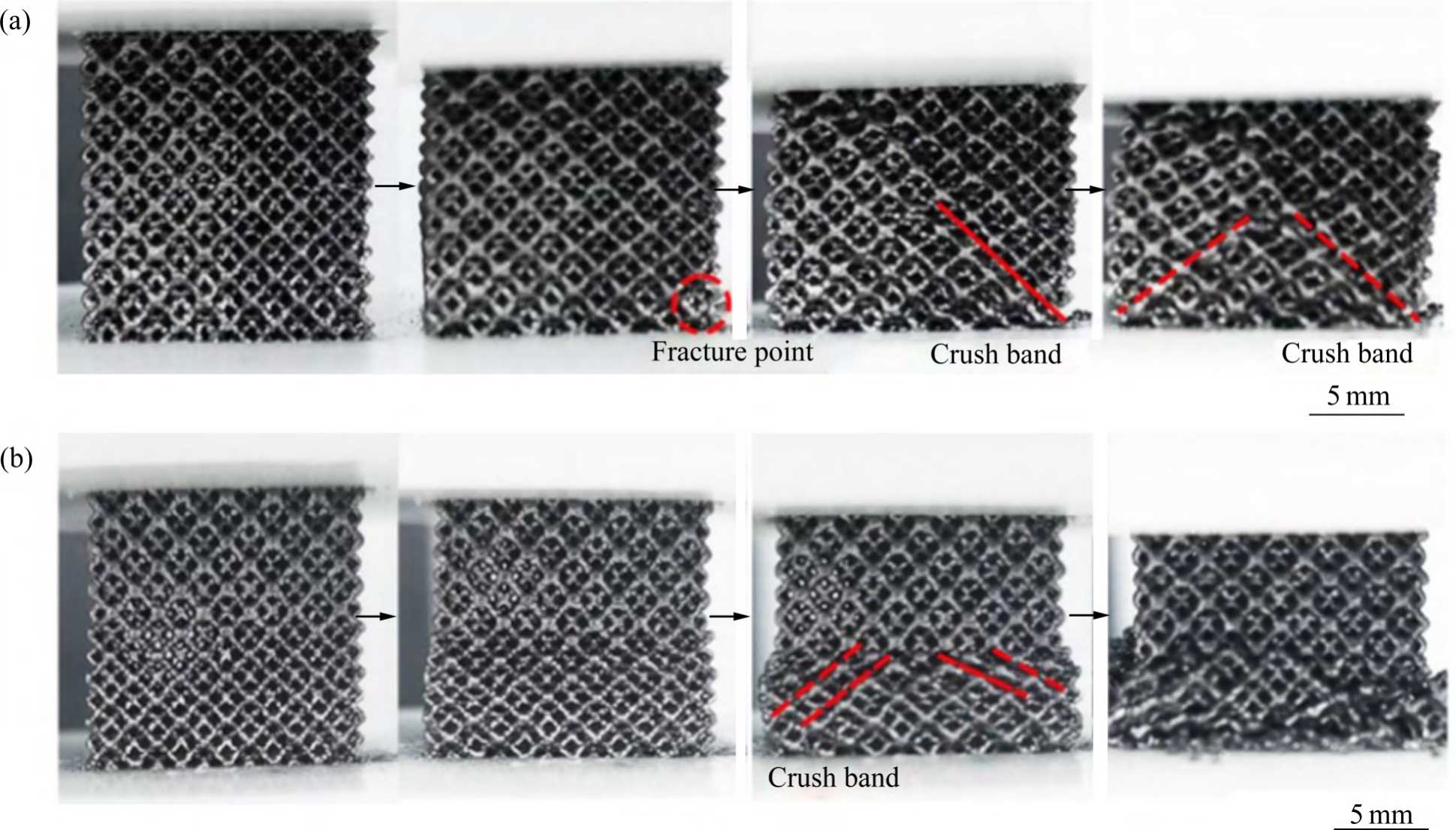
Fig. 11 Macro observations of graded tantalum scaffold during fatigue test: (a) VG; (b) PG
In addition, GUO et al [67] investigated the compressive fatigue behaviour and compressive fatigue strength of vertical gradient (VG) and parallel gradient (PG) porous tantalum. In fatigue tests, the failure of PG porous tantalum was concentrated in the lowest strength component, leading to higher ratchet strains and fatigue damage strains (see Fig. 11 [67]). In VG porous tantalum, due to the simultaneous and uniform deformation of each structure, the accumulation of strain in a single cycle is small, leading to low cyclic ratchet strains. Stress redistribution retards the propagation of fatigue cracks in high-strength porous structures, thereby reducing the fatigue damage strain. Therefore, the design idea of hierarchical pore structure under the same strain conditions is conducive to obtaining porous tantalum scaffolds with high compressive fatigue strength, which provides new ideas for the design and preparation of high-performance porous tantalum implants.
3.2 Biological properties
The pore structure parameters of porous tantalum are important factors affecting the mechanical and biological properties of its implants, including pore unit morphology, pore diameter, filament diameter, porosity, internal connectivity and so on. It is generally believed that porosity and pore diameter are highly correlated with mechanical properties (including elastic modulus, strength, etc.), permeability, and biological properties (including cell adhesion, proliferation, differentiation, inward bone growth, and osseointegration, etc.), and that higher porosity has a lower elastic modulus, which promotes the transport of nutrients and inward bone growth, but at the same time weakens the mechanical support of porous tantalum [82].
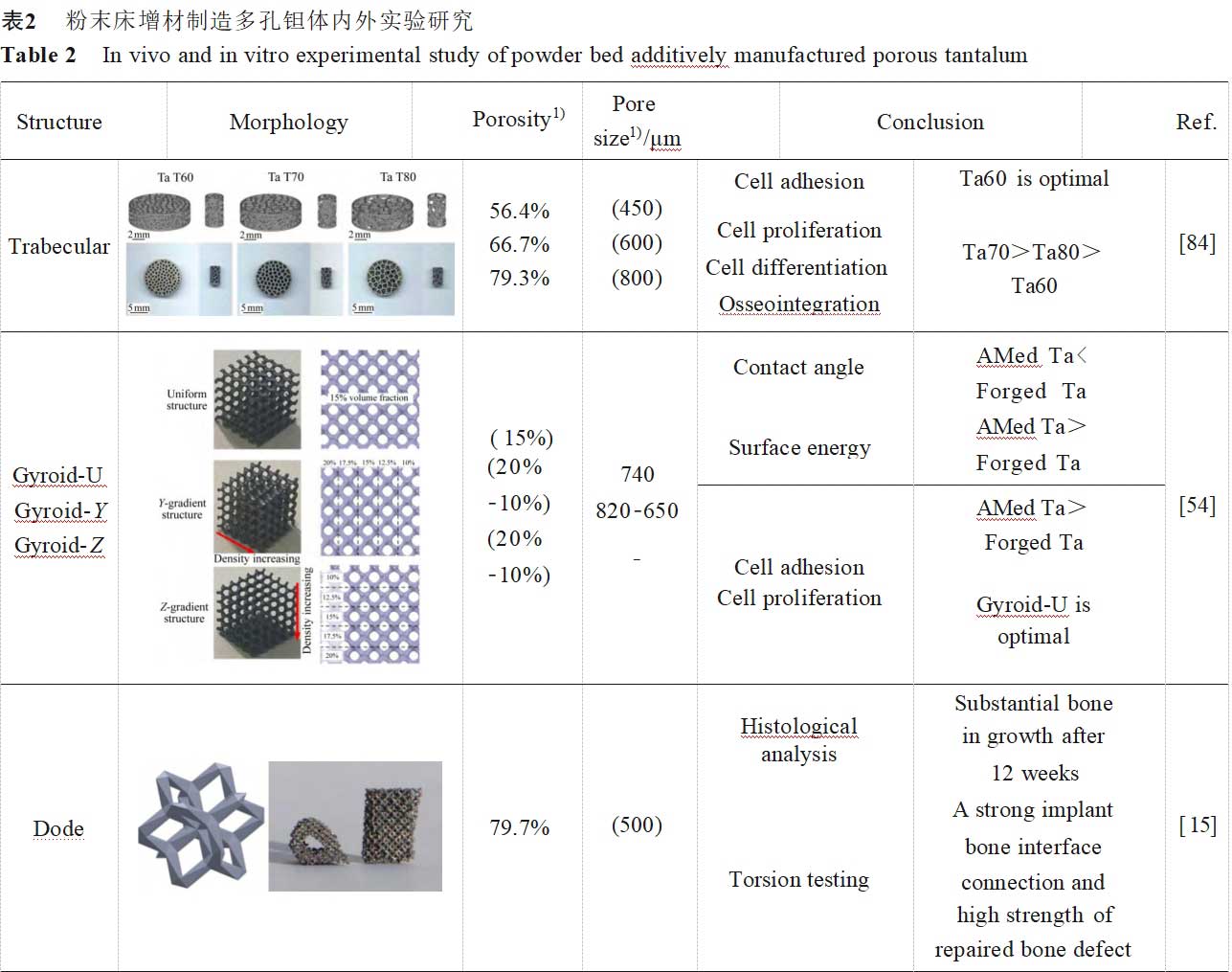
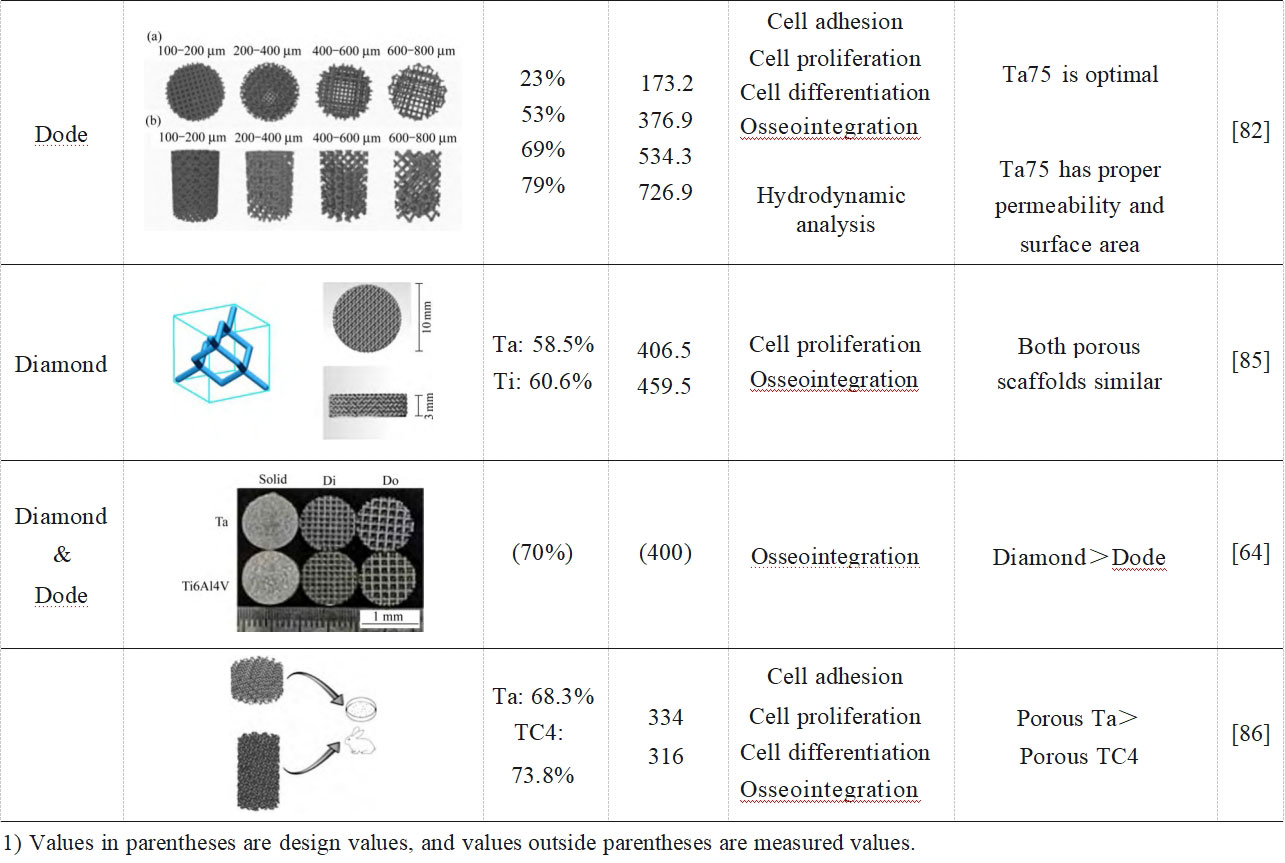
Due to differences in preparation processes, pore structure design and measurement methods, the optimal combination of pore size and porosity parameters has not yet been derived from in vivo and ex vivo studies of porous tantalum. A well-accepted conclusion is that the average pore size needs to be greater than 300 μm to support vascularisation [83], and the most successful porous implant for clinical application is the porous tantalum with bone trabecular structure from Jetmax in the USA, which has a porosity of 75%-85% and an average pore size of 400-600 μm [4]. Therefore, for the emerging additive manufacturing and the process characteristics and pore structure design freedom that this technology brings, it is crucial to investigate different combinations of pore structure parameters to find the optimal additively manufactured porous tantalum scaffolds for orthopaedic use. Table 2 [15,54,64,82,84-86] summarises the in vivo and ex vivo experimental studies of porous tantalum pore structures fabricated by powder bed additive manufacturing, which provides a new basis for further applications of additively manufactured porous tantalum orthopaedic implants.
4 Clinical applications of additively manufactured porous tantalum
In the past two decades, porous tantalum implants crowned with TrabecularMetalTM (TrabecularMetalTM from Jetmax, USA) have been widely used in the whole body parts of human hip, knee and spine. The characteristics of porous tantalum are similar to cancellous bone, and its elastic modulus matched to bone tissue, adequate mechanical support, long fatigue life, excellent corrosion resistance and osseointegrative ability ensure its application prospects in fields such as orthopaedics and dentistry. With the development of additive manufacturing technology, additively manufactured porous tantalum has achieved clinical applications in several sites by virtue of its individualised advantages, and Table 3 [53,87-91] summarises the clinical applications of additively manufactured porous tantalum in the hip, knee and other sites.

1) Values in parentheses are design values, and values outside parentheses are measured values.
5 Outlook
In terms of regulatory science and common technology research, the design and production of personalised porous tantalum implants requires the acquisition, transfer, use and storage of data, and biomedical additive manufacturing porous tantalum should be scientifically regulated based on considerations such as compliance, safety, traceability and standardisation. No evaluation system for biomedical additive manufacturing porous tantalum materials has been established, failing to identify the key properties of tantalum powder and porous tantalum implants, with many testing items and high quality control costs. Low-cost, rapid, on-site testing techniques for key parameters/performances are yet to be developed, thus facilitating traceability and improving QC. A mass spectrometer for rapid chemical characterisation of solid materials, developed by Exum in 2023 and capable of trace level detection of the entire periodic table of elements, is shown in Figure 12 [92]. LIFT USA claims that this testing equipment will be beneficial for future work in everything from powder testing to component design, manufacturing and testing of new materials, as traditional laboratory environments are often inefficient, have a limited range of elemental detection as well as often requiring external testing [92]. Less common research on additively manufactured porous tantalum, coupled with the high price of tantalum, further raises the threshold for the design and development of porous tantalum implants and their commercial application, which will be further promoted with the establishment of guidelines, standards and the development of testing technologies.
In terms of porous tantalum product design, the development of topology optimisation and analogue simulation technologies [93] facilitates the design and manufacture of porous tantalum implants with clinically required mechanical and biological properties. In addition, constraining the additive manufacturing process features into the design will enable the design of porous tantalum implants to take into account manufacturability and better realise the desired bone-like porous structure.

Fig. 12 Photo of the first laser ablation laser ionisation time-of-flight mass spectrometer Massbox
In terms of porous tantalum additive manufacturing equipment and process optimization, the current research on personalized additive manufacturing of porous tantalum is mainly evaluated from a clinical perspective, and the impact of complex personalized porous tantalum implants on manufacturing, detection and other impacts need to be further investigated, such as the fidelity of the morphological characteristics of the porous structure, and the risk of metal powder residue in the connecting interface of porous-dense composite tantalum implants. The control of microstructure in the additive manufacturing process is the key to achieving biomedical functions of porous tantalum, so there is an urgent demand for equipment functions based on artificial intelligence or machine learning for process parameter optimisation, in-situ monitoring and defect prediction, and in the future, additive manufacturing equipment and tantalum powder specifications may become more specialised according to the limiting characteristics of the porous structure and the differences in manufacturing precision.
In terms of service evaluation of additively manufactured porous tantalum, there is a lack of long-term clinical results for additively manufactured porous tantalum implants, and clinical efficacy needs to be further demonstrated. Simulating real service conditions, evaluating the fatigue life and failure mode of porous tantalum implants, and establishing standards for performance test conditions can truly reflect whether the required performance requirements are met and guide future innovative designs.
In order to improve the biological properties of porous tantalum implants, it is necessary to explore novel surface modification techniques, such as antimicrobial coatings, biomimetic calcium phosphate coatings, micro-arc oxidation, and surface functional modification to further improve the antimicrobial and osteogenic properties of porous tantalum. In addition, the mechanical properties of additively manufactured porous tantalum implants are expected to be further improved to meet the load-bearing requirements through techniques such as microstructure optimisation, tantalum plating on the surface of high-performance metal material scaffolds such as additively manufactured porous titanium alloys, and multi-material additive manufacturing. Figure 13 preliminarily explores the design and development process of additively manufactured porous tantalum implants and future research directions.
6 Conclusion
Porous tantalum implants prepared by the traditional CVD process have been widely used in clinical practice for more than two decades, demonstrating excellent corrosion resistance, biocompatibility and osseointegration, proving the safety and effectiveness of porous tantalum. The research of additively manufactured porous tantalum has gone deeper from the development of process parameters to the defect mechanism and reinforcement mechanism, continuously optimising the design of porous tantalum pore structure through multi-faceted evaluations of mechanical properties and in vitro and in vivo biology, and has already been clinically applied in multiple sites by virtue of its personalised advantages. In addition, some new porous tantalum additive manufacturing processes are being developed, including additive manufacturing of porous titanium alloy scaffold surface plating of tantalum, titanium-tantalum multi-material additive manufacturing and additive manufacturing of new porous titanium-tantalum alloys and so on. With the booming development of additive manufacturing technology, the clinical application of porous tantalum is expected to continue to expand in the future.
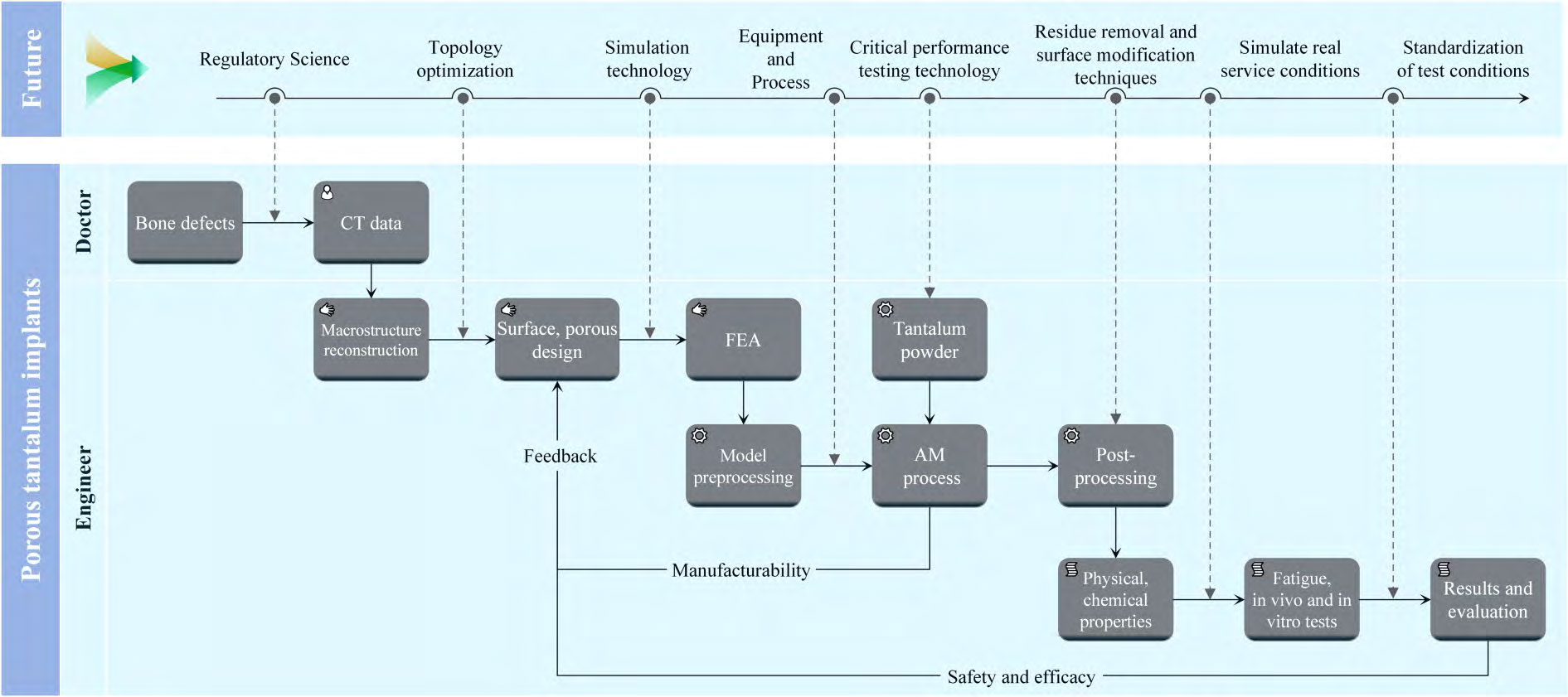
Fig. 13 Additive manufacturing porous tantalum design development process and future research directions
Reference: Chinese Journal of Nonferrous Metals Huixian Lin, et al: Research Progress of Porous Tantalum for Biomedical Additive Manufacturing
Spherical tantalum powder produced by Stardust Technology using RF plasma spheronisation technology is widely used in 3D printing of clinical medical implants for spine, joints, trauma and other applications due to its excellent biocompatibility and bone growth-in characteristics. Stardust is the first to achieve breakthroughs in the industrialisation of medical-grade spherical tantalum powder in China, and has worked with domestic first-class orthopaedic hospitals, medical device companies and other companies to promote the clinical and medical applications of tantalum metal, and has participated in the formulation of two national standards related to GB/T 38975-2020 Tantalum and Tantalum Alloy Powder for Additive Manufacturing, GB/T 41883-2022 Tantalum and Tantalum Alloy for Powder Bed Melt Additive Manufacturing, and YY/T 1851-2022 for additive manufacturing of medical pure tantalum powder industry standard 1, T/CAMDI 065-2021 additive manufacturing of tantalum metal knee prosthesis, T/CAMDI 066-2021 additive manufacturing of tantalum metal individualised bone defect filler and other group standards 3, to assist in completing the clinical application of tantalum metal for more than 500 cases, implantation of prosthetics, including intervertebral fusion of tantalum metal, bone implanted prostheses include tantalum metal intervertebral fusion device, bone cushion, hip joint, shoulder joint, knee joint, ankle joint and so on.
Hotline: +86 13318326187 Cathie Zheng
