
[Abstract] Objective To study the effect of porous tantalum and porous titanium implants on bone integration. Methods By computer-aided design method, two porous material implants with the same microporous parameters were prepared by 3D printing technology: porous tantalum and porous titanium. A bone defect model was established at the bilateral femoral lateral malleolus of 24 New Zealand white rabbits. The left and right defects of each animal were randomly divided into two groups and repaired with porous tantalum (experimental group) and porous titanium (control group) implants, respectively. Samples were collected 2, 4, and 8 weeks after implantation for gross observation and methylene blue-acid fuchsin staining to observe the bone integration of the implant and bone interface, and the push-out test was used to test the bonding strength of the implant-bone interface. Results At 2, 4, and 8 weeks after surgery, the new bone tissue at the interface of the two groups gradually increased, and new bone trabeculae appeared and grew into the pores of the materials; there was no statistically significant difference in the osteogenesis and implant-bone tissue interface bonding strength between the two groups (P>0.05). Conclusion 3D printed porous tantalum can form early biological bonding with bone tissue and has bone integration ability comparable to porous titanium.
The elastic modulus of the dense implants currently used is significantly higher than that of human bone tissue. After implantation, stress shielding and stress concentration can be generated, causing degeneration and absorption of bone tissue around the implant, and even leading to implant loosening. In order to promote bone integration, reduce the elastic modulus, and eliminate stress shielding, bone implants usually use a porous surface layer to give them good osteoconductivity, thereby inducing bone ingrowth to achieve biological fixation and ensure the long-term stability of the implant [1-3]. At present, two types of porous metal materials are commonly used for the porous surface of bone implants: porous titanium and porous tantalum. Porous titanium is generally processed by bead blasting, powder and other processes, while porous tantalum is processed by vapor deposition or powder metallurgy [3-5]. In vitro studies have found that compared with porous titanium, bone marrow mesenchymal stem cells grown on porous tantalum have stronger cell adhesion and proliferation abilities, and higher expression levels of osteogenic related indicators such as alkaline phosphatase and osteogenic related transcription factor 2 [6]. Due to the different processing techniques of porous titanium and porous tantalum, it is difficult to ensure that the two porous materials have the same microporous structural parameters (porosity, pore size, wire diameter, pore type, spatial arrangement) to compare the bone ingrowth and bone integration performance of the two. In recent years, with the increasing maturity of 3D printing technology, the microporous configuration of porous titanium and porous tantalum can be precisely controlled, so that porous materials can be prepared on the basis of the same design configuration, and the two can be compared under the condition that the microporous structural parameters are basically the same. The purpose of this study is to prepare porous tantalum and porous titanium implant specimens based on 3D printing technology, and to explore the bone ingrowth and bone integration ability of porous titanium and porous tantalum under the same microporous structural parameters by implanting them into the rabbit femoral lateral malleolus bone defect model, so as to provide a theoretical basis for the research and application of new porous implants.
1 Materials and Methods
1.1 Experimental Animals
Healthy adult New Zealand white rabbits aged 6 months were selected as experimental animals, of either sex, with a body weight of 2.5-3.5 kg, provided by the Experimental Animal Center of Chongqing Medical University and kept in the animal room of the Key Laboratory of the Affiliated Stomatological Hospital of Chongqing Medical University. The experimental plan was approved by the Animal Ethics Committee of Chongqing Medical University, and all animals were managed in accordance with the guidance and use guidelines of the National Animal Laboratory.
1.2 Main instruments and reagents
S-3000N scanning electron microscope (SEM; Hitachi, Japan), C-SAILOR dental implant machine (COXO, Yusen Medical Equipment Co., Ltd., Foshan, Guangdong, China), E300CP/400CS hard tissue cutting and grinding system (EX AKT Vertriebs GmH, Germany), TCS.SP8 laser scanning confocal microscope (Leica, Germany), C43.104 electronic universal testing machine (MTS, Shenzhen Meters, China). Sodium pentobarbital, methylene blue, acid fuchsin (Sigma, USA).
1.3 Design and production of porous implants
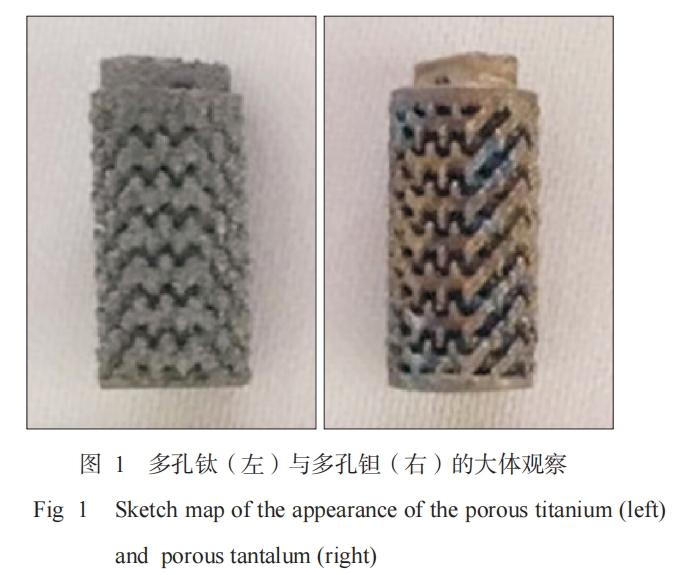
A cylinder with a diameter of 4.8 mm, a length of 8 mm, a pore size of 400 μm, and a porosity of 70% was designed by computer aided design (CAD) modeling. Porous titanium and porous tantalum implants were prepared by 3D printing technology (produced by Zhuzhou Printe Additive Manufacturing Co., Ltd., Figure 1). They were ultrasonically cleaned in distilled water, acetone solution, and 70% ethanol solution for 30 min, respectively, and then washed with distilled water and sterilized with high-pressure steam for use.
1.4 Experimental grouping and methods
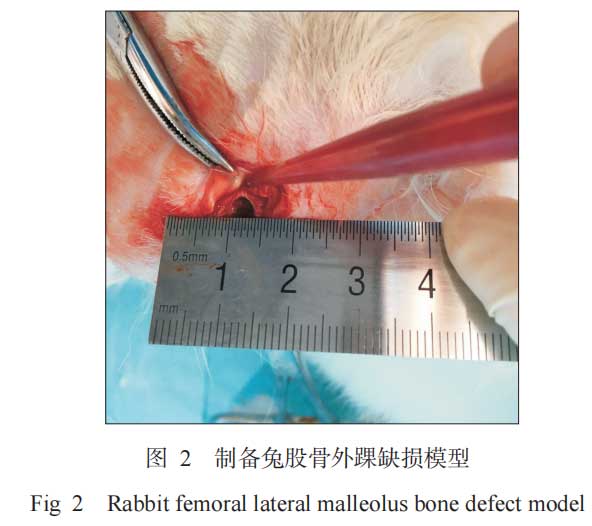
Bone defects were prepared at the lateral malleolus of the left and right femurs of experimental animals. The left and right sides of each animal were randomly divided into two groups, and one porous tantalum (experimental group) and one porous titanium (control group) were implanted, respectively. A total of 48 implants were implanted, namely 24 in the experimental group and 24 in the control group. Twenty-four New Zealand rabbits were divided into three groups according to the time nodes, namely 2 weeks, 4 weeks and 8 weeks. The 2-week group and the 4-week group each included 6 animals, and the 8-week group included 12 animals, of which 6 were used for push-out test and 6 were used for histological examination. The specific methods of bone defect and implant surgery are as follows. 3% sodium pentobarbital was used for general anesthesia through the marginal ear vein at a dose of 1 mL kg-1. After the anesthesia was effective, the skin around the lateral malleolus of both lower limbs was prepared. According to the aseptic surgical operation specifications, the surgical area was disinfected with 1% povidone-iodine and the drape was laid routinely. A longitudinal incision of about 2 cm was made at the lateral condyle of the femur. The skin was cut open, and the subcutaneous tissue and muscle were separated layer by layer to the periosteum. The periosteum was peeled off to expose the lateral condyles of the femur on both sides. A cylindrical bone defect cavity with a depth of 8 mm and a diameter of 4.8 mm was drilled at the center of the femoral condyle 4 mm away from the distal articular surface using a dental drill (Figure 2). According to the experimental groups, porous tantalum and porous titanium with a diameter of 4.8 mm and a length of 8 mm were implanted in the defects of the bilateral femoral lateral malleolus of rabbits, respectively. After flushing again, the wounds were sutured layer by layer, and penicillin 800,000 U was injected intramuscularly once a day for 5 consecutive days after surgery to prevent infection in the surgical area. The animals were kept and observed under normal conditions, and the postoperative wound infection was closely observed. Ten days after surgery, the wound sutures were removed. Each group of experimental animals was killed by ear vein air embolism at 2, 4, and 8 weeks after surgery, and bilateral femoral specimens were taken for testing.
1.5 Observation indicators
1.5.1 Observation of implant surface morphology Before implantation, SEM was used to observe the implant surface morphology and compare the differences in the surface morphology of the two materials.
1.5.2 Test of mechanical properties of implants The compressive strength of porous titanium and porous tantalum was measured by an electronic universal testing machine, and the corresponding elastic modulus was calculated based on the measured results. The number of samples used for mechanical testing was 3 parallel samples.
1.5.3 Gross observation Observe the bone integration of the two groups of materials with the host at each time point after surgery with the naked eye.
1.5.4 Tissue morphological observation Six animals were killed at each time point after surgery. The femoral specimens obtained were fixed, dehydrated, infiltrated, embedded, and sliced. The planes with complete exposure of the materials were stained with methylene blue-acid fuchsin, and the formation of new bone tissue and bone formation around the implanted materials were observed under an optical microscope. The bone ingrowth area ratio is defined as the ratio of the actual area of bone ingrowth to the total area of pores in the porous material that can provide tissue ingrowth. Two fields of view were randomly selected for each slice. The vertical distance from the deepest bone tissue growth to the implant interface in the field of view was the bone growth depth. Image-Pro Plus 6.0 software was used to calculate the bone growth area rate and bone growth depth of the stained slices.
1.5.5 Implant-bone interface bonding strength The push-out method was used to test the bonding strength between the implant and the bone tissue. Six animals in each group were killed 8 weeks after surgery. The bones containing the samples were cut with diamond pieces and the push-out test was performed on an electronic universal testing machine. The loading rate was 0.5 mm·min-1. The maximum push-out force was used to represent the bonding strength between the sample and the surrounding tissue.
1.6 Statistical analysis
SPSS 17.0 statistical software was used. The measurement results were expressed as arithmetic mean ± standard deviation. The t test was used for statistical testing. The test level was bilateral α=0.05.
2 Results
2.1 Material appearance structure and morphological characteristics
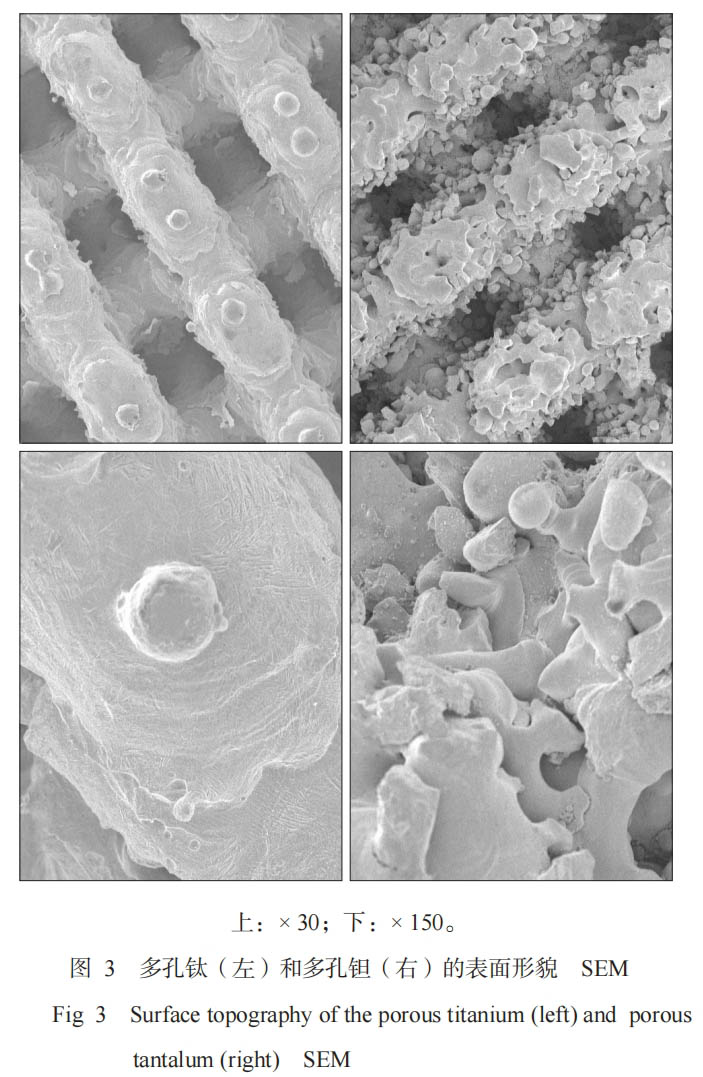
The surface and cross section of porous titanium and porous tantalum show uniformly distributed three-dimensional honeycomb microporous structures, and the pores of the micropores are interconnected. The surface of porous titanium is smoother than that of porous tantalum (Figure 3).
2.2 Mechanical properties test results
The mechanical properties test results show that the compressive strength of porous titanium and porous tantalum are (395.60±0.80) and (138.10±6.88) MPa respectively; the elastic modulus is (5.454±0.060) and (3.104±0.107) GPa respectively, which are both lower than the value of cortical bone, and the porous tantalum is smaller than that of porous titanium, and its elastic modulus is closer to cancellous bone than that of porous titanium.
2.3 Gross observation
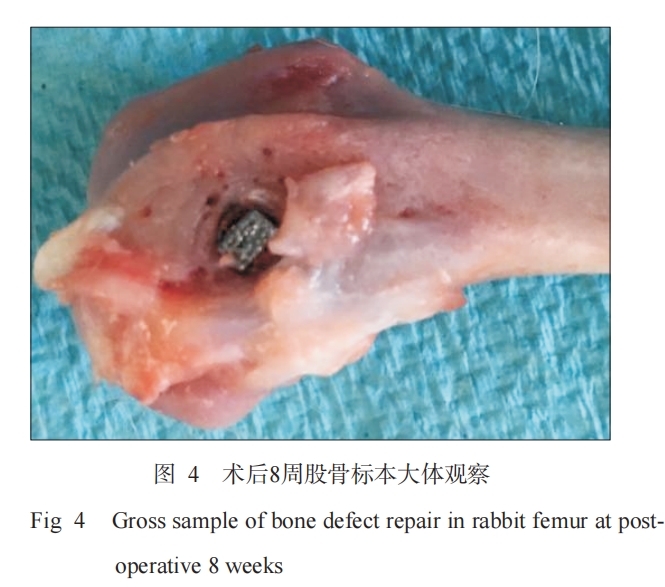
The surgery of all animals was successfully completed, and they woke up naturally within 2 hours after surgery. There were no signs of infection such as redness, swelling, and exudation in the surgical wound. Six days after surgery, the experimental animals basically resumed normal activities. At each observation time point after surgery (2, 4, and 8 weeks), the two groups of materials were well integrated with the surrounding bone tissue, and no phenomena such as detachment and displacement were observed (Figure 4).
2.4 Histological observation
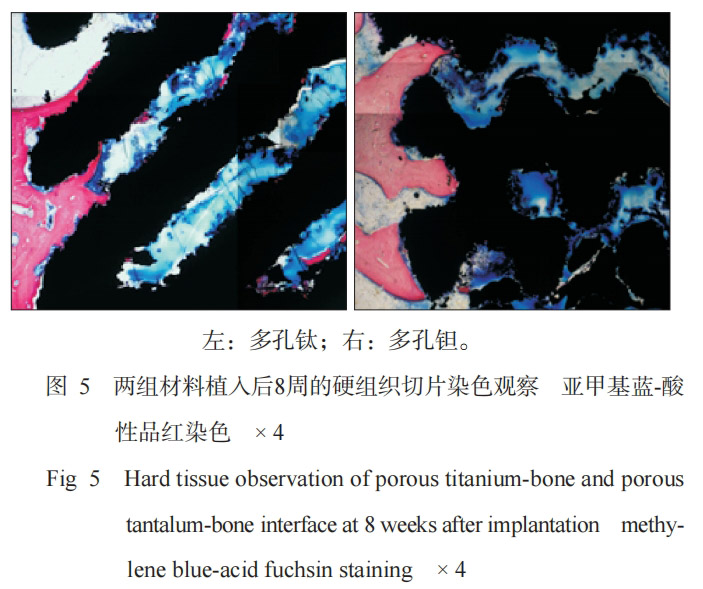
Methylene blue-acid fuchsin staining (Figure 5) showed that at 8 weeks after surgery, the two groups of materials were closely integrated with mature bone tissue, and the bone tissues were continuous with each other. At 8 weeks, the bone ingrowth area rates of porous titanium and porous tantalum were 19.35%±2.35% and 17.98%±1.81%, respectively, and the bone ingrowth depths were 51.59±1.68 and 49.25±0.85, respectively (in pixels). According to statistical analysis, there was no significant difference in the bone ingrowth area rate and bone ingrowth depth between the two groups of materials (P>0.05).
2.5 Implant-bone interface bonding strength
The results of the push-out test showed that the average maximum push-out force of the porous titanium and porous tantalum samples was (411.30±37.03) and (382.70±60.05) N, respectively, and there was no statistically significant difference between the two groups (P>0.05).
3 Discussion
In this study, the microporous design parameters of porous titanium and porous tantalum samples were the same (wire diameter 400 μm, pore size 400 μm, porosity 70%). Existing studies [7] have confirmed that porosity, pore size and interconnectedness of pores are key factors affecting the mechanical and biological properties of porous implants. Therefore, it is very important to manufacture porous materials with suitable pore structure and mechanical properties. Generally, pore sizes between 100 and 600 μm are suitable for bone tissue growth, while pore sizes greater than 200 μm are conducive to the migration of capillary tissue and osteoprogenitor cells, and are beneficial to bone tissue generation [8]. 3D printing technology can achieve precise control of porous parameters such as porosity and pore size of porous materials, so that the parameters of the porous materials can be compared with each other to maintain consistency [9]. This study observed the microscopic porous morphology of the two groups of specimens. The results showed that the porous titanium surface was smoother than the porous tantalum (Figure 3). The reason is that the melting point of tantalum is too high (about 2996 °C), resulting in the presence of unmelted tantalum metal particles during processing. Rønold et al. [10] found that the roughness of the sandblasted surface is conducive to the mechanical integration of bone tissue on the implant surface, improves the stability of the initial implantation, is conducive to the adhesion, proliferation and differentiation of surface osteoblasts, and is more conducive to bone formation. Biomechanical studies generally believe that "stress shielding" can lead to bone resorption and affect the long-term effect of bone integration. When designing and manufacturing implants, both biocompatibility and mechanical properties should be considered [3,11]. The mechanical test results of this study showed that the elastic moduli of porous tantalum and porous titanium were (3.104±0.107) and (5.454±0.060) GPa, respectively, which were between cancellous bone and cortical bone, significantly lower than the traditional orthopedic implant material titanium alloy (110 GPa), and close to the elastic modulus of human bone, which is consistent with the experimental results of foreign scholars [7]. However, the difference between the elastic moduli of porous tantalum and porous titanium was statistically significant, and the elastic modulus of porous tantalum was smaller, indicating that porous tantalum may better avoid "stress shielding" than porous titanium and provide effective mechanical stimulation for bone tissue [3,12].
Given that in vivo experiments can accurately reflect the true state of the material's bone integration ability, this study used animal distal femur implant materials to explore the effects of the two groups of materials on bone integration. The hard tissue sections were stained with methylene blue-acid fuchsin, and it can be seen that the bone bonding interface of the sample is clear, and red bone tissue can be seen. The bone tissue outside the implant may be mature bone tissue before implantation, while the bone tissue inside the implant cavity is newly grown. Both porous titanium and porous tantalum samples obtained good bone ingrowth histological evaluation, which was 19.35%±.35% and 17.98%±1.81%, respectively. The new bone tissue was evenly distributed inside the two implants, which may be attributed to the good interconnection between the pores, thereby promoting the growth of surrounding bone tissue.
This experiment used a push-out test to evaluate the bone bonding strength of the implant-bone interface. In the push-out test, the damage first occurred at the bonding interface between the implant and the bone rather than the implant itself. The maximum push-out forces of porous tantalum and porous titanium were (382.70±0.05)
and (411.30±37.03) N, respectively, with no significant difference between the two, indicating that the bone bonding performance of the two is comparable. After the porous structure is introduced into the implant, the newly formed bone tissue can grow into the pores of the porous implant, so that the implant and bone tissue form a biological fixation. The porous material can increase the contact area between the bone and the implant. In addition, the mechanical interlocking effect between the bone and the porous pores increases the shear strength of the implant and bone interface accordingly [13-14].
Porous tantalum has a three-dimensional interconnected microporous structure, which gives it good physical properties such as low elastic modulus and high surface friction coefficient. Studies [15] have shown that during the loading process, traditional implants can absorb 30% of the load energy, while porous tantalum implants can absorb 50%~75%. Because of the higher friction coefficient, it has good initial stability during the implantation process, thereby improving the success rate of the implant surgery. Although the porous structure can effectively reduce the elastic modulus of the implant, it also reduces the fatigue strength of the implant, making it more likely to break than dense implants, and it is relatively difficult to prepare and shape. How to find a balance between them still needs further research.
In summary, in this study, porous titanium and porous tantalum have the same excellent bone ingrowth and bone integration capabilities, but the elastic modulus of porous tantalum is closer to human bone and has better biomechanical adaptability. The results of this study confirm that, at least in short-term implantation studies, the bone integration performance of 3D printed porous tantalum specimens is not inferior to that of porous titanium specimens, which can provide experimental basis for 3D printing personalized implants; but it is undeniable that the biomechanical properties of porous tantalum specimens are better, and the elastic modulus close to human bone may be beneficial to its long-term implant stability. Of course, this still needs more in-depth long-term observation and fatigue test and other related research.
About Stardust Technology
Stardust Technology (Guangdong) Co., Ltd. is a national high-tech enterprise specializing in the research, development, production and sales of high-end spherical powder materials for 3D printing, powder metallurgy, surface engineering and other fields. The company insists on taking radio frequency plasma spheroidization powder making technology as the core, and provides internationally advanced powder products and application solutions.
The company's main products include high-end rare refractory metals such as tungsten, molybdenum, tantalum, niobium, vanadium, rhenium, chromium and their alloys, compound spherical powders. At the same time, it provides technical services such as radio frequency plasma spheroidization, plasma rotating electrode atomization, 3D printing, hot isostatic pressing, injection molding, powder metallurgy, etc.
Medical grade spherical tantalum powder
The spherical tantalum powder produced by Stardust Technology using radio frequency plasma spheroidization technology is widely used in 3D printing of clinical medical implants such as spine, joints, and trauma due to its excellent biocompatibility and bone ingrowth characteristics. Stardust Technology is the first in China to achieve an industrial breakthrough in medical-grade spherical tantalum powder. It has jointly promoted the clinical medical application of tantalum metal with domestic first-class orthopedic hospitals and medical device companies. It has participated in the formulation of GB/T 38975-2020 Tantalum and tantalum alloy powder for additive manufacturing, GB/T 41883-2022 Powder bed fusion additive manufacturing of tantalum and composite alloys related national standards, YY/T1851-2022 Medical pure tantalum powder for additive manufacturing industry standard 1, T/CAMDI065-2021 Additive manufacturing tantalum metal knee prosthesis, T/CAMDI 066-2021 Additive manufacturing tantalum metal individualized bone defect filler "and other group standards, and assisted in the completion of more than 500 cases of tantalum metal clinical application, implanted prostheses include tantalum metal intervertebral fusion bone spacers, hip joints, shoulder joints, knee joints, ankle joints, etc.
https://www.stardustpowder.com/spherical-pure-ta-powder

Welcome to inquire

Paper citation information
West China Journal of Stomatology Vol. 36 No. 3 June 2018