
Introduction
Tungsten possesses a high melting point, high hardness, and excellent corrosion and radiation resistance, offering broad application prospects in the field of thermonuclear fusion. Particularly within the International Thermonuclear Experimental Reactor (ITER) project, tungsten is regarded as an ideal material for plasma-facing components [1-2]. However, its brittleness at room temperature limits its processing and application. Currently, pure tungsten components are primarily fabricated using powder metallurgy techniques [3], yet these methods struggle to meet modern industrial demands for complex structures, high precision, and multifunctional integrated components. Laser powder bed fusion (LPBF) technology, characterized by high design freedom and the ability to produce integrated parts, offers a new viable approach for structural optimization of first wall and target plate components [4-5]. Nevertheless, due to tungsten's high melting point, elevated ductile-to-brittle transition temperature, and low laser absorption rate [6-7], laser powder bed fusion of pure tungsten requires high energy input. This high energy input readily induces temperature gradients in the melt pool, forming nanoscale textured structures that enhance surface energy and laser absorption. This technique provides an effective pathway for achieving low-energy-density laser powder bed fusion of pure tungsten. TERTULIANO et al. [10] discovered that for low-laser-absorption metals like copper and aluminum, wet etching precisely controls nanostructure morphology and distribution. This increases surface area while enhancing laser-powder interaction, enabling high-quality forming at low energy densities. BATISTÃO et al. [11] observed that acid and alkali etching treatments elevated powder laser absorption rates to 30.7% and 35.3%, respectively, while significantly improving powder flowability.
Currently, domestic research on surface activation of high-purity spherical tungsten powder for laser additive manufacturing remains in its infancy. The authors employed acid etching (hydrofluoric acid) and alkaline etching (hydrogen peroxide) methods to activate the surface of high-purity spherical tungsten powder. They investigated the effects of these two processes on the surface morphology and properties of the tungsten powder, aiming to provide process support and theoretical basis for high-quality pure tungsten components fabricated via laser powder bed fusion at low energy densities.
1. Sample Preparation and Experimental Methods
The experimental raw material comprised high-purity spherical tungsten powder produced via radiofrequency plasma spheroidization. Its chemical composition (mass fraction/%) was 0.001Fe, 0.001Cu, 0.011O, 0.0003Ni, 0.003C, 0.001P, balance W, supplied by Beijing AVIC Mate Technology Co., Ltd. Its microstructure is shown in Figure 1, exhibiting spherical particles with smooth, uniform surfaces and an average particle size of 18.68 μm. Hydrofluoric acid, analytical grade, with HF mass fraction ≥40%, supplied by Shanghai Aladdin Bio-Chem Technology Co., Ltd. Ammonium fluoride, analytical grade, supplied by Shanghai Aladdin Bio-Chem Technology Co., Ltd. Hydrogen peroxide, analytical grade, with a mass fraction of hydrogen peroxide not less than 30%, supplied by Shanghai Sigma-Aldrich Trading Co., Ltd.

Surface activation of spherical tungsten powder was performed using two processes: acid etching and alkali etching, referred to as acid activation and alkali activation, respectively. The acid etching process flow is as follows: Following the methodology from the author's research group's prior studies [12-13], measure 440 mL of deionized water into a beaker. Sequentially add 1 g of ammonium fluoride and 60 mL of hydrofluoric acid, then stir thoroughly using a magnetic stirrer. Add 5 g of tungsten powder and perform ultrasonic dispersion combined with mechanical stirring for 30 min. After standing for 30 min, remove the supernatant. Rinse three times with deionized water and anhydrous ethanol, respectively, then dry in a 60°C oven. The alkaline etching process was designed based on the protocol reported by TERTULIANO et al. [10], with the specific procedure as follows: Measure 20 mL hydrogen peroxide into a 500 mL Erlenmeyer flask. Add 80 g tungsten powder and stir ultrasonically and manually. Observe the solution color change from colorless to yellowish-brown, accompanied by significant exothermic heat and gas release within a short time. Once the reaction stabilizes with no noticeable bubble formation, decant the supernatant. After repeating the above process 1–2 times, wash the residue three times with deionized water and anhydrous ethanol, respectively, then dry at 60°C.
The surface morphology of the powder was observed using a Gemini 500 high-resolution thermo-field emission scanning electron microscope (SEM), and micro-area compositional analysis was performed using the energy dispersive spectrometer (EDS) attached to the SEM. Particle size distribution was measured using an MS-2000 laser diffraction particle size analyzer. Characteristic size parameters D10, D50, and D90 were selected at cumulative volume fractions of 10%, 50%, and 90%, respectively. The particle size distribution width was characterized using the irregular distribution index kIDR, calculated as follows: kIDR = (D90 - D10) / D50 (1)
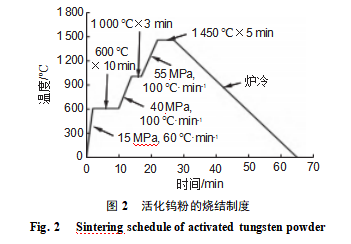
Phase composition analysis was performed using a Bruker D8 Advance ECO X-ray diffractometer (XRD) with a copper target, Kα radiation, an operating voltage of 40 kV, a scanning range of 10°–90°, and a scanning rate of 10 (°)·min^(−1). Following GB/T 1482—2022 “Determination of Flowability of Metal Powders—Standard Funnel Method (Hall Flowmeter)”, the Hall flowmeter model DGL-H002T was used to measure the time t50g required for 50 g of activated powder to pass through a standard funnel with a 2.5 mm aperture. This value characterizes powder flowability, with the average taken from seven parallel tests. Currently, lasers in commonly used laser powder bed fusion equipment are primarily infrared fiber lasers with wavelengths typically ranging from 1060 to 1100 nm [14-15]. Therefore, a Cary 5000 UV-Vis-NIR spectrophotometer was employed to determine the laser absorption rate of the powder at wavelengths between 1060 and 1100 nm using diffuse reflectance spectroscopy. An LABOX-350 discharge plasma sintering furnace was used to sinter the activated powder into bulk specimens under vacuum conditions, with a vacuum level of approximately 1×10^(−5) Pa. The sintering conditions are shown in Figure 2. The sintered specimens were ground and polished, and their densities were measured using the Archimedes displacement method. Relative densities were calculated based on the theoretical density of pure tungsten (19.3 g·cm^(−3)). The microstructure of the sintered specimens was observed using an Axio Lab A 1 optical microscope (OM).
2. Experimental Results and Discussion
2.1 Effects on Powder Surface Properties and Particle Size Distribution
As shown in Figure 3: Only diffraction peaks characteristic of body-centered cubic (BCC) tungsten (PDF#04-0806) were detected in the tungsten powder prior to activation. Compared to unactivated tungsten powder, the number and positions of diffraction peaks in acid-activated tungsten powder remained unchanged. However, the diffraction peaks corresponding to the (211) and (220) crystal planes exhibited significant broadening and slightly enhanced intensity. This indicates that acid activation did not alter the crystal structure but introduced significant lattice distortion and microstrain. primarily due to the non-uniform etching of the near-surface region of tungsten particles by hydrofluoric acid, leading to increased defect density.
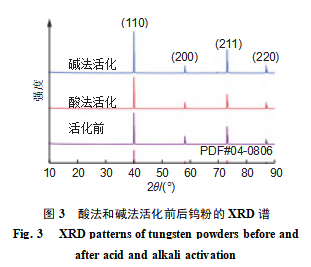
After alkaline activation, the diffraction peaks of tungsten with a BCC structure remain predominant. However, the intensity of the (110) crystal plane diffraction peak significantly increases and its shape becomes sharper. Simultaneously, a few unidentified weak diffraction peaks appear near 2θ = 70°, This is primarily due to the anisotropic etching of tungsten crystal planes by hydrofluoric acid. Within the BCC crystal structure, the {110} plane exhibits the densest atomic arrangement and lowest surface energy, resulting in a significantly slower etching rate compared to the {100} and {111} planes. Consequently, it is preferentially retained during activation, leading to enhanced diffraction peak intensity.
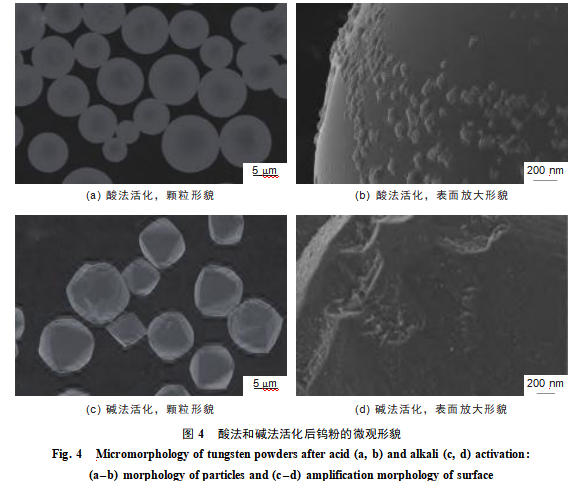
As shown in Figure 4, the acid-activated tungsten powder particles retain their original spherical morphology. This is because during acid activation, the soluble hexafluorotungstate complex ([WF6]2−) formed by the complexation reaction between hydrofluoric acid solution and tungsten dissolves rapidly, preventing sustained localized corrosion and thus preserving the integrity of the spherical structure. only localized areas exhibit nanoscale protrusions. After alkaline activation, the sphericity of tungsten powder particles decreases, showing slight deformation and adopting an octahedral-like morphology. This occurs because the {100} and {111} crystal planes have relatively loose atomic packing and higher surface energy, leading to preferential corrosion under hydrogen peroxide action. In contrast, the {110} crystal plane features dense atomic arrangement and lower surface energy, allowing this plane to be preserved, ultimately forming an octahedral morphology dominated by {110} planes. The surface developed a uniform, dense structure of nano-scale protrusions, with smaller protrusion dimensions, indicating that alkali activation has a greater impact on the surface morphology of tungsten powder particles than acid activation. After acid activation, localized agglomeration occurred in the tungsten powder.
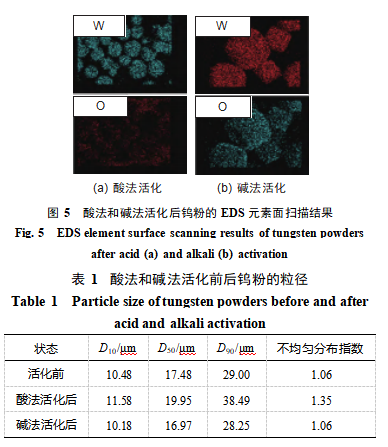
As shown in Figure 5: After acid activation, the oxygen content on the tungsten powder surface is extremely low (mass fraction of 1.12%). Combined with XRD analysis, it indicates that almost no oxide impurities were introduced. After alkaline activation, a small amount of oxygen enrichment appears on the tungsten powder surface (oxygen mass fraction of 6.04%), and impurity peaks appear in the XRD spectrum. It is speculated that a small amount of tungsten oxide was formed and deposited on the tungsten powder surface during the alkaline activation process.
Table 1 shows that acid activation increases the particle size of tungsten powder. This may be related to trace residual F⁻ ions or complexes on the surface. F⁻ ions and complexes weaken electrostatic repulsion between particles, inducing slight agglomeration and resulting in an apparent increase in particle size. Alkaline activation slightly reduces the particle size of tungsten powder. Following acid and alkaline activation, the uniformity indices of the tungsten powder were 1.35 and 1.06, respectively. A lower uniformity index indicates a more concentrated particle size distribution and improved uniformity. Evidently, alkaline activation resulted in a more uniform particle size distribution, which enhances the stability of powder spreading and improves forming quality during laser powder bed fusion.
2.2 Effects on Powder Flowability and Laser Absorption Rate
The time t50g required for 50 g of unactivated tungsten powder, acid-activated tungsten powder, and alkali-activated tungsten powder to pass through a standard 2.5 mm aperture funnel was measured as 5.40, 18.47, and 5.82 s, respectively. It is evident that: the flowability of unactivated tungsten powder is good; Compared to the unactivated state, acid activation significantly reduced flowability, primarily due to localized agglomeration of tungsten particles; alkali activation caused a slight decrease in flowability, possibly related to increased interparticle contact area and friction resulting from the octahedral-like structure of the tungsten powder.
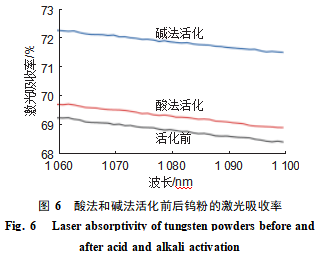
Figure 6 shows that within the tested wavelength range, the laser absorption rate of tungsten powder decreased with increasing wavelength for both activated and unactivated samples; Both acid and alkali activation enhanced the laser absorption rate of tungsten powder, with alkali activation yielding a higher absorption rate. The infrared laser of the BLT-S210 laser additive manufacturing equipment operates at approximately 1070 nm. At this wavelength, the laser absorption rates for acid-activated and alkali-activated tungsten powders were 69.60% and 72.15%, representing increases of 0.49% and 3.04% respectively compared to the pre-activation value (69.11%). The activation process formed nanoscale protrusions on the powder surface, increasing surface roughness. This enhanced multiple light scattering, reduced reflection losses, and improved laser absorption efficiency. The alkaline-activated tungsten powder exhibited more uniform and finer-sized nanoscale protrusions, resulting in superior laser absorption performance.
2.3 Effect on Powder Sintering Properties
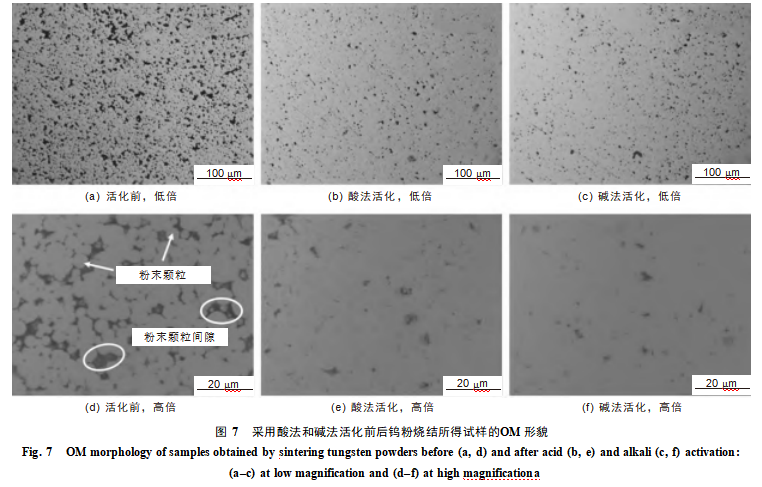
Figure 7 shows that sintered specimens prepared from unactivated tungsten powder exhibit observable powder particles and numerous pores (circled in the figure). Specimens sintered from acid-activated and alkali-activated tungsten powders display significantly reduced porosity with no discernible powder particles. Calculations show the relative densities of the sintered specimens from unactivated tungsten powder, acid-activated tungsten powder, and alkali-activated tungsten powder were 93.38%, 95.97%, and 96.24%, respectively. This indicates that surface activation treatment enhances the relative density and compactness of the sintered specimens. This is attributed to the introduction of nanostructures on the tungsten powder particle surfaces via acid/alkali activation, which increases surface energy. This, in turn, lowers the diffusion energy threshold during sintering and promotes uniform fusion between particles. The alkali-activated particles exhibit more uniform and denser nanostructures on their surfaces, resulting in superior enhancement of sintering density.
3. Conclusion
(1) After hydrofluoric acid etching (acid activation), tungsten powder particles retained a spherical shape with localized agglomeration. Nanoscale protrusions appeared in specific surface areas, and no significant impurities were introduced. Following hydrogen peroxide etching (alkali activation), particles transformed into an octahedral-like structure with slightly reduced particle size. Uniform, denser, and smaller nanoscale protrusions formed on the surface, accompanied by trace amounts of tungsten oxide.
(2) The flow times of 50 g acid-activated and alkali-activated tungsten powder through a standard 2.5 mm sieve were 18.47 s and 5.82 s, respectively, both exceeding that of unactivated tungsten powder (5.40 s). Surface-activated tungsten powder exhibited reduced flowability. Compared to unactivated tungsten powder, acid- and alkali-activated tungsten powders exhibited enhanced laser absorption in the infrared wavelength range commonly used for laser powder bed fusion. At 1070 nm, laser absorption increased to 69.6% and 72.15% for acid- and alkali-activated powders, respectively.
(3) The relative densities of sintered specimens prepared from acid-activated and alkali-activated tungsten powders increased to 95.97% and 96.24%, respectively, with improved sintering density after surface activation. Alkali activation demonstrated superior effects on enhancing tungsten powder's laser absorption rate and sintering performance compared to acid activation.
Reference: MATERIALS FOR MECHANICAL ENGINEERING Vol. 49, No. 11; Effects of Acid and Alkaline Etching on Surface Morphology and Properties of Spherical Pure Tungsten Powder for Laser Powder Bed Fusion; Zhang Xiaoqiang 1,2, Liu Jiaqin 3,4, Zhu Xiaoyong 2,5, Wu Yucheng 1,2,4
The influence of acid and alkali etching on the surface morphology and properties of spherical pure tungsten powder for laser powder bed fusion is essentially a key technological step in optimizing powder flowability, wettability, and fusion quality during the 3D printing process. Based on spherical powders of rare refractory metals and centered on 3D printing and hot isostatic pressing services, Stardust Technology's integrated solution not only provides a scientific pathway for optimizing the surface properties of spherical pure tungsten powder but also achieves full-chain quality control spanning “powder preparation - surface treatment - 3D printing.” Looking ahead, as aerospace, nuclear power, semiconductors, and other high-end sectors demand increasingly advanced properties from refractory metals, this integrated collaborative model of “precision material performance control” will become a core driver of industrial advancement. Stardust Technology will continue deepening technological innovation, seamlessly integrating products and services to deliver more efficient and superior spherical powder solutions and 3D printing services for rare refractory metals. For further product details, please contact our Sales Manager, Sukie Zhu, at +86 13378626726.
